Abstract
Functionally 4,6-disubstituted 1H-pyrazolo[3,4-d]pyrimidines are important compounds with various pharmacological activities. 1-Substituted 4-chloro-6-(chloromethyl)-1H-pyrazolo[3,4-d]pyrimidines are practically unexplored derivatives in this series. In this paper, it was shown that the nucleophilic substitution of 4-Chloro-6-(chloromethyl)-1-methyl-1H-pyrazolo[3,4-d]pyrimidine with methylamine produced selectively 4-substituted product, 6-(chloromethyl)-N,1-dimethyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine. The structure of the synthesized compound was established by elemental analysis, high resolution mass-spectrometry, 1H, 13C-NMR, and IR spectroscopy, mass-spectrometry, and X-ray analysis.
1. Introduction
1H-Pyrazolo[3,4-d]pyrimidines have attracted great interest as purine analogs. They exhibited various biological activities, including anti-inflammatory [1], antiproliferative [2], antifungal [3], and many other activities [4]. Functionally 4,6-disubstituted 1-phenyl-1H-pyrazolo[3,4-d]pyrimidines showed good antibacterial and anticancer activity [5]. New derivatives of this class can be considered as compounds with great potential for biological activity. We recently reported on the synthesis of 4-chloro-6-(chloromethyl)-1-methyl-1H-pyrazolo[3,4-d]pyrimidine which has two centers for nucleophilic substitution of chlorine atoms [6]. Its analogue, 4-chloro-6-(chloromethyl)-1-methyl-1H-pyrazolo[3,4-d]pyrimidine, was studied in the reaction of nucleophilic substitution with amines which led to disubstituted products with equal substituents in the pyrimidine ring and 2-methylene groups [5]. To expand the range of functionally 4,6-disubstituted 1-methyl-1H-pyrazolo[3,4-d]pyrimidines it was necessary to stop the reaction of mono-substituted derivative. According to the literature, it was rather difficult to predict where the first nucleophilic substitution will occur. Herein, we report the reaction of 4-chloro-6-(chloromethyl)-1-methyl-1H-pyrazolo[3,4-d]pyrimidine with methylamine and the regioselective synthesis of 6-(chloromethyl)-N,1-dimethyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine. This compound can be considered as an important intermediate for the preparation of previously inaccessible disubstituted 1H-pyrazolo[3,4-d]pyrimidines.
2. Results and Discussion
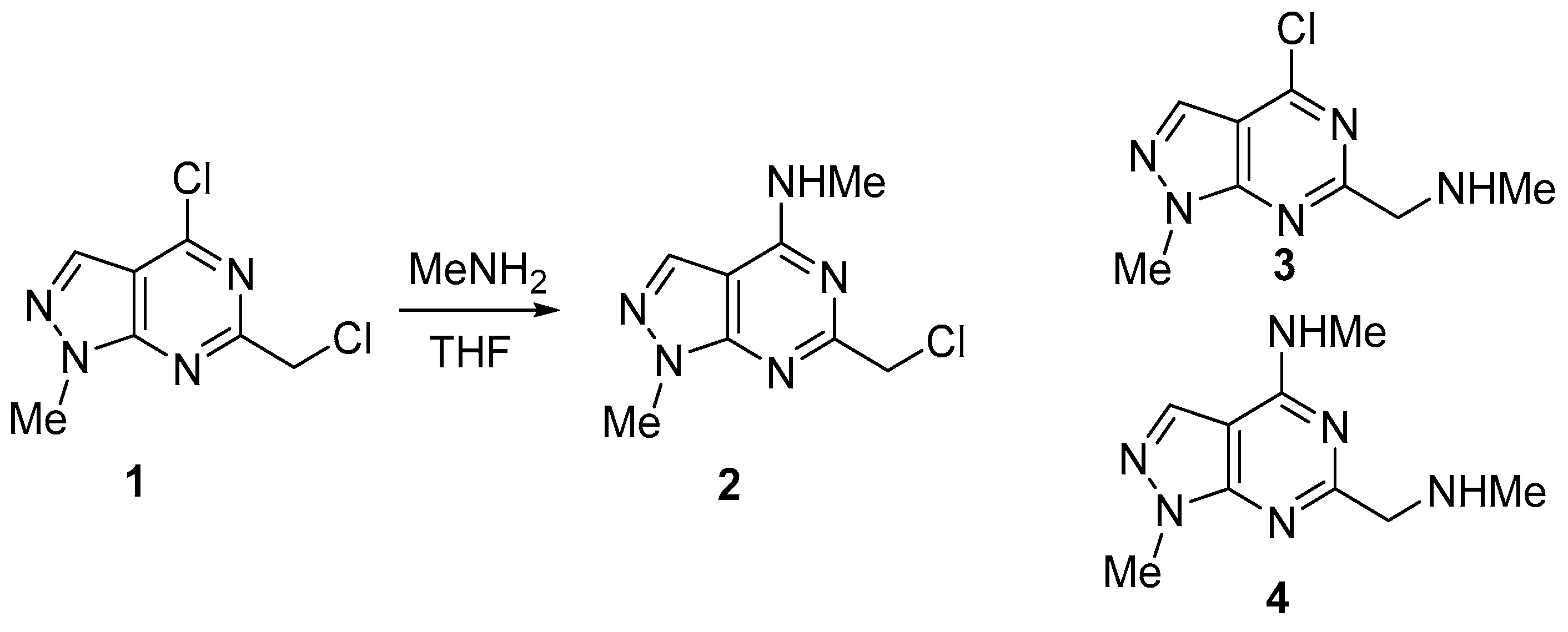
Nucleophilic substitution of 4-chloro-6-(chloromethyl)-1-methyl-1H-pyrazolo[3,4-d]pyrimidine 1, which contains two reactive chlorine atoms in the 4 position of pyrimidine ring and in CH2Cl group with methylamine was examined in this paper. Through this reaction, three products can be formed: two monosubstituted derivatives 2 and 3, and disubstituted compound 4. Treatment of dichloro derivative 1 with two equivalent of MeNH2 (one for nucleophilic substitution and one equivalent for binding the released HCl) in THF at rt led to the formation of only product 2 with a high yield of 71% (Scheme 1). Neither the starting compound 1, nor the substitution product in the CH2Cl group 3, nor the disubstitution product 4 were found in the reaction mixture. It turned out that the process is highly selective, and that the replacement of the chlorine atom in 4-position of pyrimidine ring does occur.
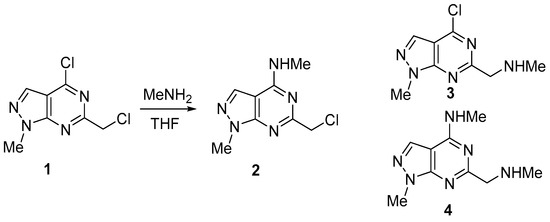
Scheme 1.
Synthesis of 6-(chloromethyl)-N,1-dimethyl-1H-pyrazolo[3,4-d] pyrimidin-4-amine 2.
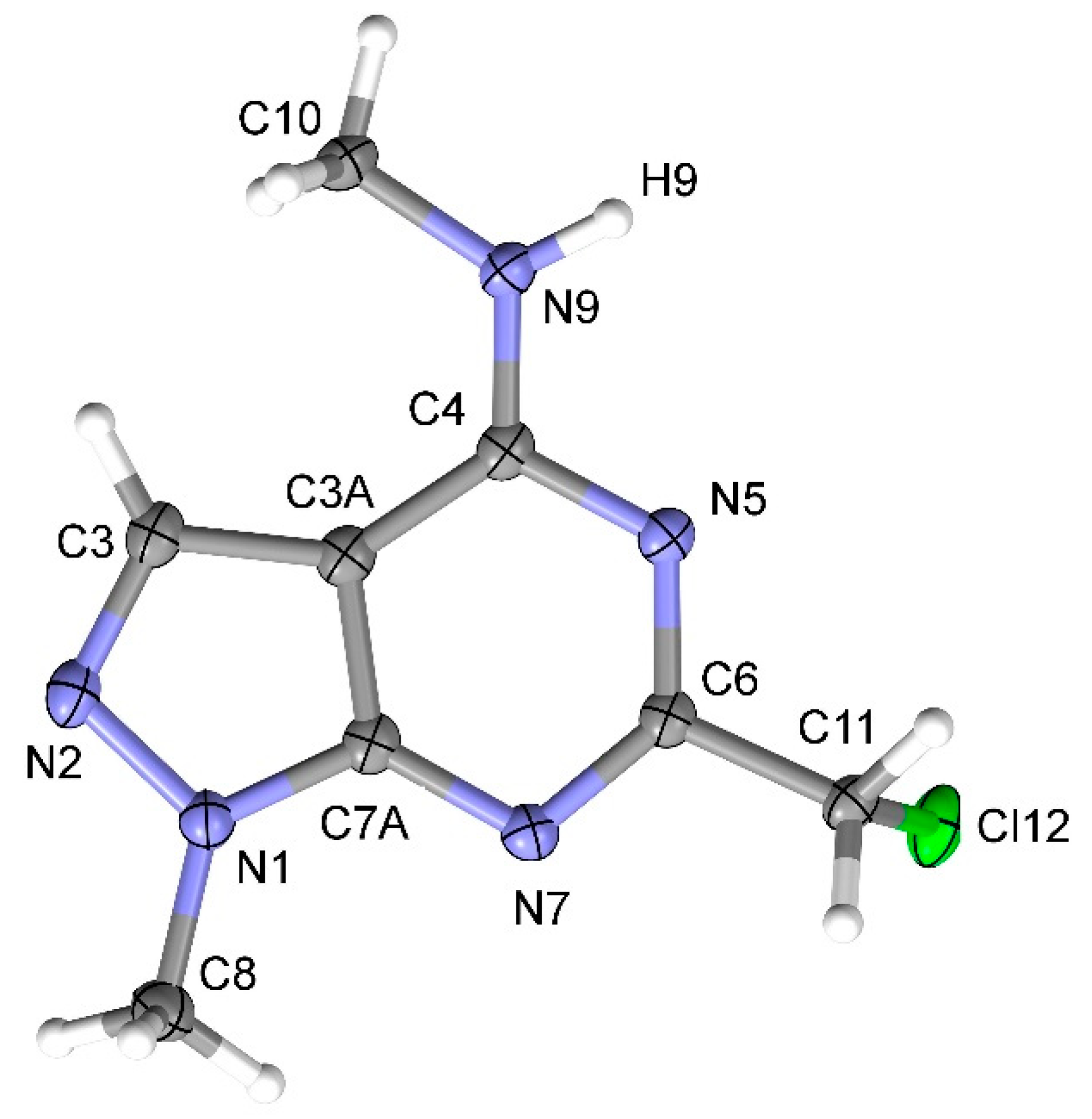
The structure of 6-(chloromethyl)-N,1-dimethyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2 was fully confirmed by elemental analysis, high resolution mass-spectrometry, 1H, 13C-NMR and IR spectroscopy, and mass-spectrometry. The 1H-NMR spectrum of 2 showed singlets of the two Me group (3.14 and 3.96 ppm), RCH2 group (4.61 ppm) and C-H-pyrazole group (8.00 ppm) which can be characteristic of mono-substituted derivatives 2 or 3. Finally, the structure of 4-methylamino derivative 2 was proved by X-ray diffraction analysis (Figure 1).
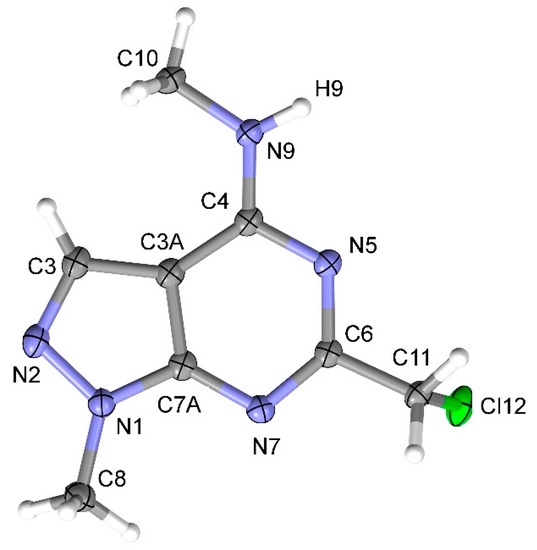
Figure 1.
X-Ray structure (ORTEP at 50% level) of 6-(chloromethyl)-N,1-dimethyl-1H-pyrazolo[3,4-d] pyrimidin-4-amine 2.
In conclusion, it was shown that the nucleophilic substitution of 4-chloro-6-(chloromethyl)-1-methyl-1H-pyrazolo[3,4-d]pyrimidine 1 with methylamine is regioselective and led to 4-methylamino derivative 2. This compound is a convenient precursor for various functionally substituted 1-methyl-1H-pyrazolo[3,4-d]pyrimidines, which may be of interest as substances with useful pharmacological properties.
3. Materials and Methods
4-Chloro-6-(chloromethyl)-1-methyl-1H-pyrazolo[3,4-d]pyrimidine was prepared according to the published method [6]. The solvents and reagents were purchased from commercial sources and used as received. Elemental analysis was performed on a 2400 Elemental Analyzer (PerkinElmer Inc., Waltham, MA, USA). Melting point was determined on a Kofler hot-stage apparatus and is uncorrected. 1H and 13C-NMR spectra were taken with a Bruker AM-300 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA) (at frequencies of 300 and 75 MHz) with TMS as the standard. MS spectrum (EI, 70 eV) was obtained with a Finnigan MAT INCOS 50 instrument (Hazlet, NJ, USA). IR spectrum was measured with a Bruker “Alpha-T” instrument in KBr pellet. High-resolution MS spectrum was measured on a Bruker micrOTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) using electrospray ionization (ESI).
6-(Chloromethyl)-N,1-dimethyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2 (CAS 1255147-44-4) is commercially available from 1Click Chemistry, Inc. (Tinton Falls, NJ, USA), 1Pluschem LLC (San Dirgo, CA, USA), A2B Chem LLC (San Dirgo, CA, USA), AA BLOCKS LLC (San Dirgo, CA, USA), abcr GmbH (Karlsruhe, Germany), Accel Pharmtech, LLC (East Brunswick, NJ, USA), Sigma-Aldrich (St. Louis, MO, USA), Aurora Fine Chemicals LLC (San Diego, CA, USA), AURUM Pharmatech, LLC (Franklin Park, NJ, USA), BIONET/Key Organics Ltd. (Cornwall, UK), Biosynth Carbosynth Limited (Berkshire, UK), ChemDiv, Inc. (San Diego, CA, USA), Chemenu Inc. (Shanghai, China), Chemieliva Pharmaceutical Co., Ltd. (Chongquing, China), Combi-Blocks, Inc. (San Diego, CA, USA), Fluorochem Ltd. (Derbyshire, UK), Heteroz, LLC (Triangle Park, NC, USA), LabNetwork, a WuXi AppTec Company (Cambridge, MA, USA), Matrix Scientific (Columbia, SC, USA), Synnovator, Inc. (Durham, NC, USA), Toronto Research Chemicals Inc. (North York, ON, Canada).
X-ray diffraction data were collected at 100 K on a four-circle Rigaku Synergy S diffractometer equipped with a HyPix600HE area-detector (kappa geometry, shutterless ω-scan technique), using graphite monochromatized Cu Kα-radiation. The intensity data were integrated and corrected for absorption and decay by the CrysAlisPro program [7]. The structure was solved by direct methods using SHELXT [8] and refined on F2 using SHELXL-2018 [9] in the OLEX2 program [10]. All non-hydrogen atoms were refined with individual anisotropic displacement parameters. The location of hydrogen atoms H9 and H29 was found from the electron density-difference map; this hydrogen atom was refined with an individual isotropic displacement parameter. All other hydrogen atoms were placed in ideal calculated positions and refined as riding atoms with relative isotropic displacement parameters. Cambridge Crystallographic Data Centre contains the supplementary crystallographic data for this paper No. CCDC 2116243. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, accessed on 19 October 2021 (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
Synthesis of 6-(chloromethyl)-N,1-dimethyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine 2 (Supplementary Materials).
Methylamine (40%, 1.7 mL, 0.02 mol) was added to the solution of pyrazolopyrimidine 1 (2.17 g, 0.01 mol) in THF (30 mL) at a temperature of 10–15 °C. The reaction mixture was stirred for 5 h at 20 °C, and the solvent was distilled. Water (20 mL) was added to the residue, the reaction mixture was stirred for 15 min and the formed precipitate was filtered off (1.68 g, 80%), crystallized from alcohol/water (1:1). Yield 1.5 g (71%), white solid, mp 170–171 °C. IR spectrum (KBr), ν, cm–1: 3435, 3252 and 3118 (all C-H, N-H), 1613, 1587 (C=N), 1444, 1357 (CH3, CH2) 1281, 988, 855, 761, 730, 683, 628. 1H-NMR (acetone-d6, ppm): δ 3.14 (3H, s), 3.96 (3H, s), 4.61 (2H, s), 7.45 (1H, br s), 8.00 (1H, s). 13C-NMR (DMSO-d6, ppm): 27.4 (NH-CH3), 34.0 (N-CH3), 48.7 (CH2Cl), 99.7 (C4), 131.7 (C3-H), 153.6 (C9), 157.5 (C5), 162.8 (C7). MS (EI, 70 Ev), m/z (I, %): 211 (M+, 100), 182 (50), 147 (100), 80 (20), 43 (20), 28 (41), 15 (23). HRMS (ESI-TOF): calcd for C8H11ClN5 [M + H]+ 212.0697; found m/z 212.0701. Anal. calcd for C8H10ClN5: C, 45.40; H, 4.76; N, 33.09; found: C, 45.36; H, 4.85; N, 33.25%.
Crystallographic data are given in Table 1.

Table 1.
Crystal data and structure refinement for compound 2.
Supplementary Materials
The following are available online: CIF file, copies of 1H, 13C-NMR, IR, HRMS, and mass-spectra for the compound 2.
Author Contributions
Synthetic experiments, analysis of experimental results, and NMR data, V.A.O.; conceptualization, writing—review and editing, supervision, and project administration, O.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Crystal structure determination was performed in the Department of Structural Studies of N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1 and 2 are available from the authors.
References
- Quintela, J.M.; Peinador, C.; Lez, L.G.; Devesa, I.; Ferrandiz, M.L.; Alcaraz, M.J.; Riguera, R. Synthesis and evaluation of trans 3,4-cyclopropyl L-arginine analogues as isoform selective inhibitors of nitric oxide synthase. Bioorg. Med. Chem. 2003, 11, 863–868. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Mohamed, M.A.; Ahmed, R.R.; Ahmed, S.A. Synthesis and anti-tumor activities of some new pyridines and pyrazolo[1,5-a]pyrimidines. Eur. J. Med. Chem. 2009, 44, 3519–3523. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Mahalinga, M.; Karthikeyan, M.S.; Akberali, P.M.; Shetty, N.S. Synthesis of some novel pyrazolo[3,4-d]pyrimidine derivatives as potential antimicrobial agents. Bioorg. Med. Chem. 2006, 14, 2040–2047. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; Al-Awadi, N.; Abdelhamid, I.A. Bicyclic 5-6 Systems: Other Four Heteroatoms 2:2. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 10, pp. 600–658. [Google Scholar] [CrossRef]
- Devarakonda, M.; Doonaboina, R.; Vanga, S.; Vemu, J.; Boni, S.; Mailavaram, R.P. Synthesis of novel 2-alkyl-4-substituted-amino-pyrazolo[3,4-d]pyrimidines as new leads for anti-bacterial and anti-cancer activity. Med. Chem. Res. 2013, 22, 1090–1101. [Google Scholar] [CrossRef]
- Ogurtsov, V.A.; Rakitin, O.A. 4-Chloro-6-(chloromethyl)-1-methyl-1H-pyrazolo[3,4-d]pyrimidine. Molbank 2021, 2021, M1253. [Google Scholar] [CrossRef]
- CrysAlisPro. Version 1.171.41.106a. In Rigaku Oxford Diffraction; Rigaku Corporation: Oxford, UK, 2021. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 229–341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).