Abstract
(E)-5-(Methoxyimino)-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one was prepared by a condensation reaction from 3,4-dihydro-1H-benzo[b]azepin-2,5-dione and O-methylhydroxylamine. The configuration at the C=N double bond was determined by X-ray crystallography.
1. Introduction
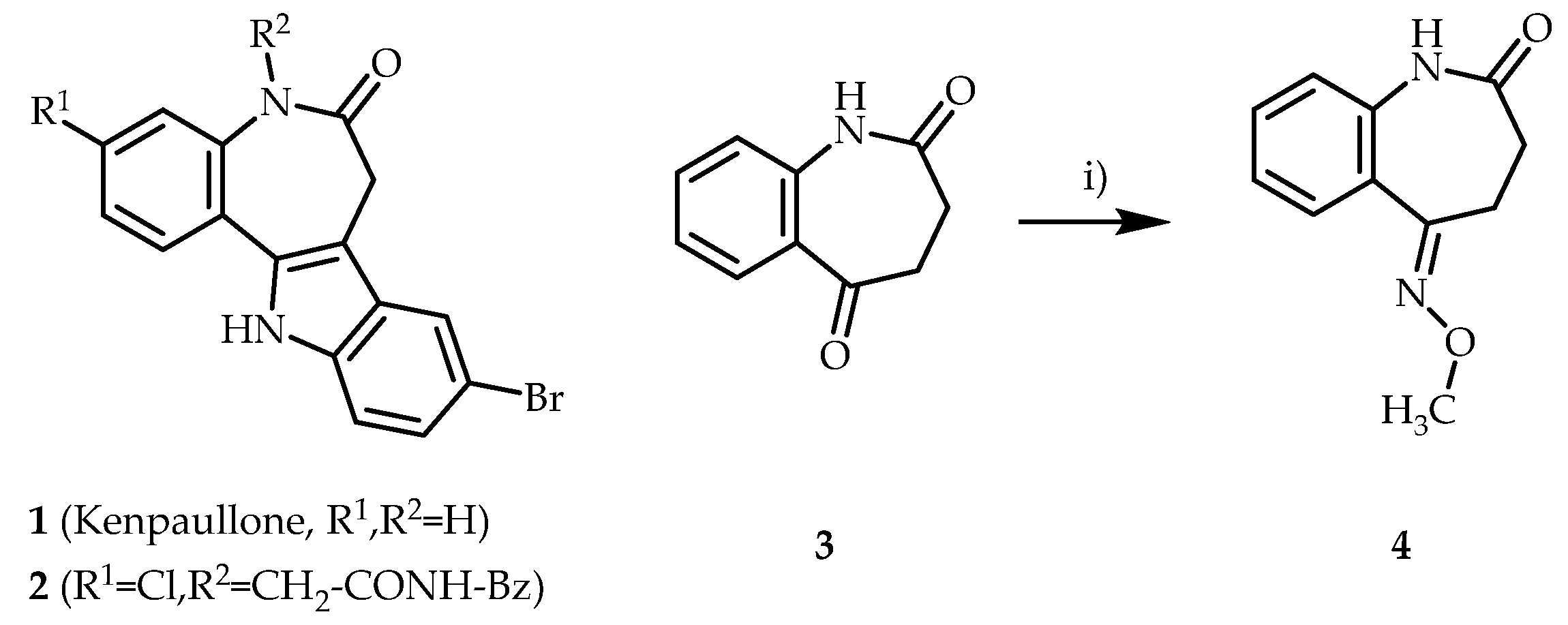
Kenpaullone (1) is the lead structure of the paullone compound family. Paullones are used as biochemical tools to inhibit cyclin-dependent kinases and glycogen synthase kinase-3 (GSK-3) [1,2,3]. Recent studies have shown that paullone-derived compounds also exhibit antiparasitic properties [4,5,6,7,8,9]. For example, the N(5)-substituted paullone 2 showed pronounced inhibition of the bloodstream form of Trypanosoma brucei brucei parasites [10]. In a project to investigate structure–activity relationships in antitrypanosomal paullones, the replacement of the indole structure in paullones with oxime ether elements was envisioned. Oxime ethers are found in various drugs such as fluvoxamine and roxithromycin [11] and especially in broad-spectrum cephalosporins, e.g., cefpodoxime proxetil [12]. In this context, the oxime ether 4 was required as a building block. The synthesis of 4, its structural characterisation, and the determination of its configuration by X-ray structural analysis are reported here.
2. Results and Discussion
The starting material 3,4-dihydro-1H-benzo[b]azepin-2,5-dione (3) [13] was refluxed with O-methylhydroxylamine and pyridine in ethanol. After precipitation, washing and drying, the desired oxime ether 4 was obtained in good yield. An initial test reaction under similar conditions but with smaller quantities of reagents (0.599 mmol of 3) led to comparable results (Scheme 1).
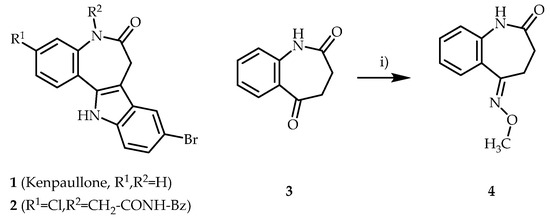
Scheme 1.
Left: Structures of kenpaullone (1) and the antitrypanosomal paullone 2. Right: Synthesis of (E)-5-(methoxyimino)-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one (4). Reagents, conditions and yields: i) pyridine, O-methylhydroxylamine, ethanol, reflux, 4–7 h, 73–74%.
The synthesis of (E)-5-(methoxyimino)-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one 4 reported here is based on a method published by Learmonth et al., who prepared the (E)-oxime of oxcarbazepine and observed the (Z)-isomer only as a minor impurity [14]. Likewise, the (E)-isomer of 4 was formed as the main product.
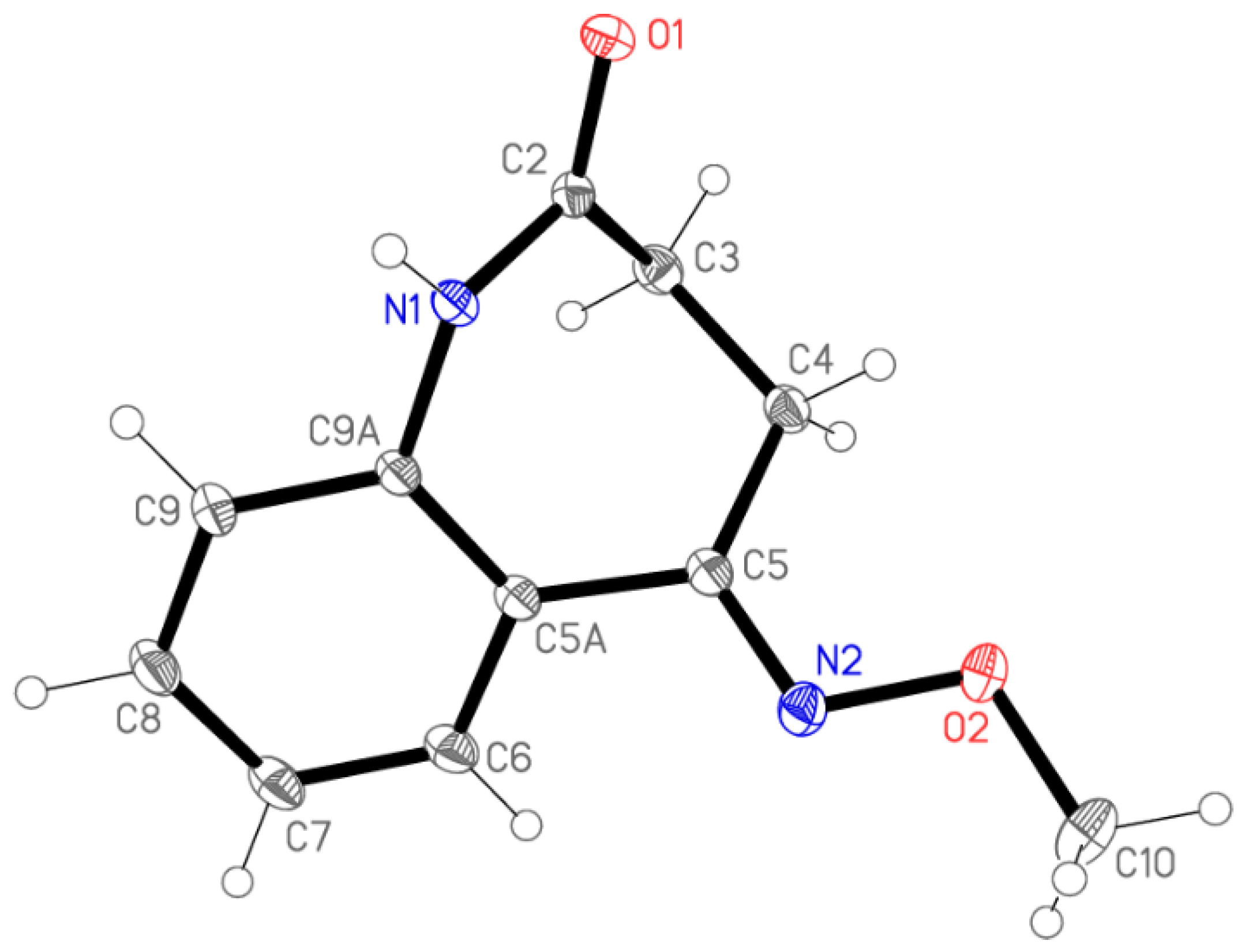
To assess the configuration at the C=N double bond, a single crystal of 4 was grown by vapour diffusion using acetonitrile as the solvent and petroleum ether as the anti-solvent. The (E)-configuration of 4 was unequivocally determined by subsequent X-ray analysis (Figure 1).
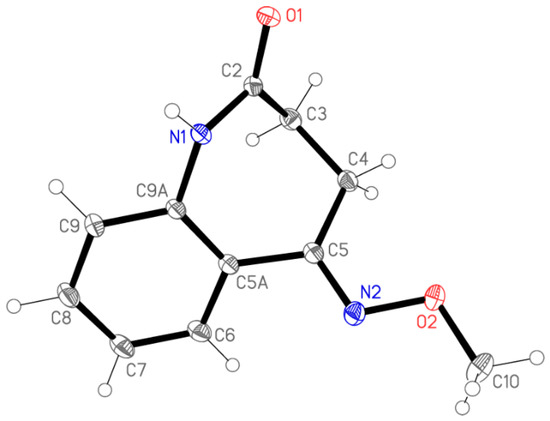
Figure 1.
The structure of compound 4 in the crystal. Ellipsoids represent 50% probability levels. Selected bond lengths [Å], bond angles and torsion angles [°]: N(2)-C(5) 1.2903(10), N(2)-O(2) 1.4133(8), C(4)-C(5) 1.5058(10), C(5)-C(5A) 1.4845(10), C(10)-O(2) 1.4315(10), C(5)-N(2)-O(2) 110.56(6), N(2)-C(5)-C(5A) 114.81(6), N(2)-C(5)-C(4) 122.93(6), C(5A)-C(5)-C(4) 122.16(6), N(2)-O(2)-C(10) 107.85(6), O(2)-N(2)-C(5)-C(5A) 177.56(6), O(2)-N(2)-C(5)-C(4) 1.00(10), C(5)-N(2)-O(2)-C(10) 179.65(6).
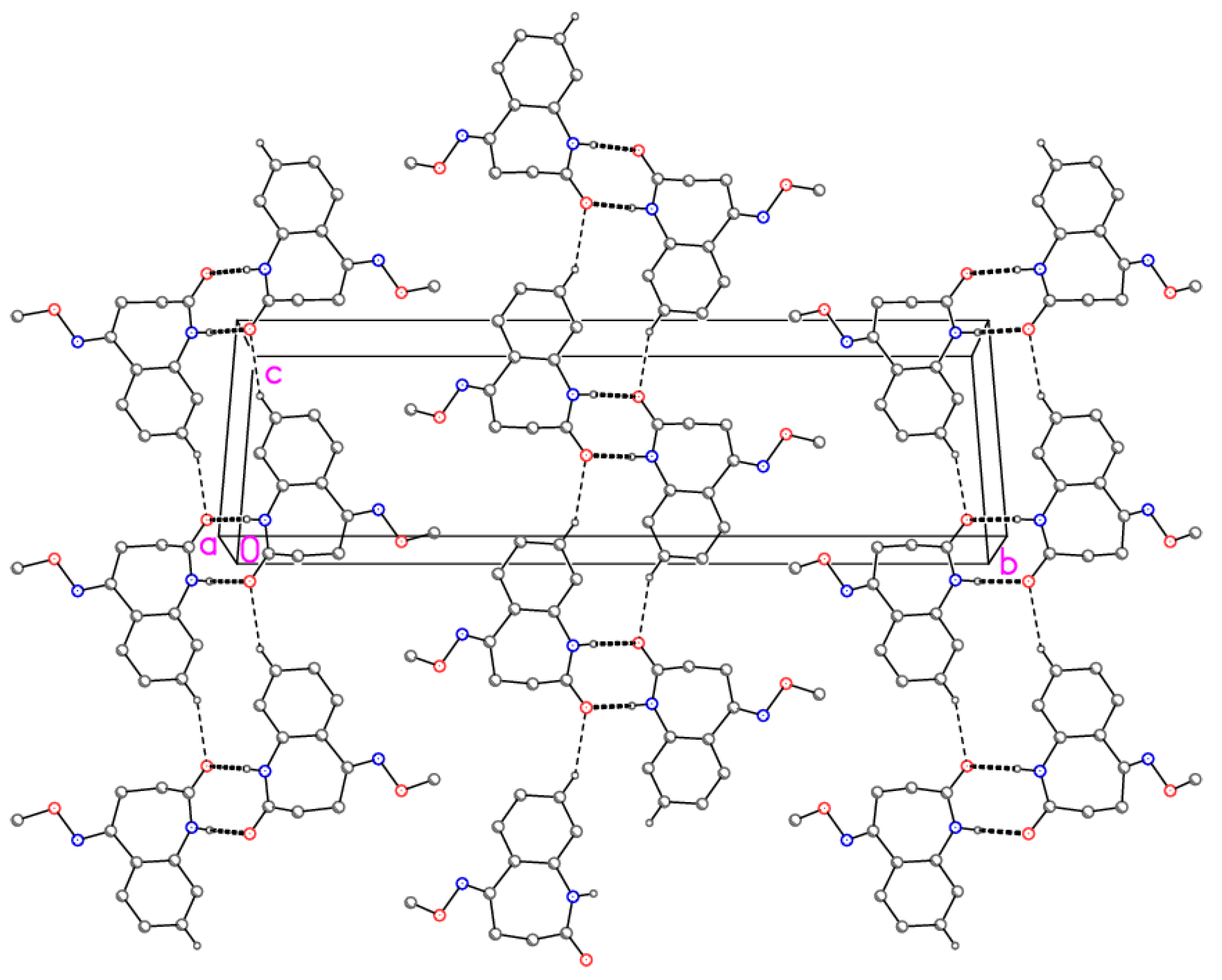
Molecular dimensions may be regarded as normal. The aromatic ring and its immediate substituents are as expected planar, with the methoxy group pointing to one side of this plane and the remainder of the azepine ring to the other side. The molecular packing involves the formation of inversion-symmetric dimers via classical hydrogen bonds N1–H⋯O1; the dimers are further linked by the “weak” hydrogen bonds C8–H8⋯O1 to form ribbons of molecules parallel to [101] (Figure 2).
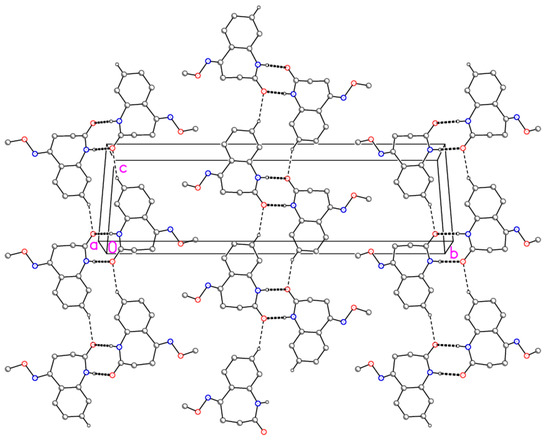
Figure 2.
Packing diagram of compound 4 viewed perpendicular to (–1 0 1). Dashed lines represent classical (thick) or “weak” (thin) hydrogen bonds. Hydrogen atoms not involved in hydrogen bonding are omitted for clarity.
The purity of 4 was assessed by elemental analysis (<0.3 per cent deviations from calculated values), HPLC (>99% at 254 nm) and 1H-NMR. The identity of the compound was confirmed by the 1H-NMR spectrum, which exhibits reasonable integrals, chemical shifts and multiplicity, e.g., the aromatic protons display two doublets of doublets and two triplets of doublets with a normalised integral value of 1, indicating an ortho-disubstituted benzene derivative. The IR spectrum shows an absorption band at 1685 cm−1 characteristic of C=N or C=O stretching. The protonated molecular ion [M + H]+ is dominant in the mass spectrum. Compound 4 will be used as a starting material for the synthesis of potential antitrypanosomal analogues of paullone 2, in which the oxime ether moiety replaces the indole ring structure.
3. Materials and Methods
3.1. Materials
Commercially available reagents were used without further purification unless otherwise stated. O-Methylhydroxylamine was purchased from Acros Organics (Geel, Belgium). Water was purified by demineralisation. HPLC solvents and reagents were of analytical grade and purchased from Sigma-Aldrich (Steinheim, Germany) or Acros Organics (Geel, Belgium); doubly distilled water was used. 3,4-Dihydro-1H-benzo[b]azepin-2,5-dione was prepared according to a published procedure [13].
3.2. Instrumentation
Melting points were measured in open-glass capillaries on an electric variable heater (Electrothermal IA 9200, Cole-Parmer, Staffordshire, UK; ramp 1 °C/min). Infrared spectra were recorded as a KBr pellet on an FTIR spectrometer (Nicolet FT-IR 200, Thermo Fisher Scientific, Waltham, MA, USA; 1/λ in cm−1; range 400–4000 cm−1). 1H-NMR and 13C-NMR spectra were recorded at 500 MHz resp. 126 MHz as a solution in DMSO-d6 on a spectrometer with a BBO probe (Avance IIIHD 500, Bruker, Billerica, MA, USA). Chemical shifts are expressed relative to the internal standard TMS (δ 0 ppm), J in Hz. C nuclei were assigned based on results from the DEPT135 experiment. HPLC was performed on a VWR Hitachi Chromaster system (Hitachi High Technologies Corporation, Tokyo, Japan) with hardware consisting of the diode array detector 5430, the column oven 5310, the pump 5110, the autosampler 5260, the column Merck LiChroCART 125-4, LiChrospher 100 RP-18 (5 μm) (Merck, Darmstadt, Germany); isocratic eluent: acetonitrile/water mixture 30:70; gradient elution: acetonitrile/water mixture with following acetonitrile concentration 0–2 min: 10%; 2–12 min: 10% → 90% (linear); 12–20 min: 90%; elution rate: 1.000 mL/min; detection wavelength: 254 nm (isocratic and gradient); overall run time: 15 min (isocratic), 20 min (gradient); tM = dead time; tMS = total retention time. For mass spectrometry, an expressionL CMS spectrometer was used with an APCI source coupled to an ASAP (atmospheric solids analysis probe) (Advion, Ltd., Harlow, UK). Measurements were performed simultaneously in the negative (−) and positive (+) ionisation modes. On-site generated nitrogen gas was used for nebulisation. Characteristic ions, adducts and fragments are subject to interpretation. The elemental analysis was performed on a CE Instruments Flash EA 1112 Elemental Analyzer (Thermo Quest, San Jose, CA, USA). TLC: Polygram SIL G/UV254, 0.2 mm thickness (Macherey-Nagel, Düren, Germany). Theoretical values were calculated using Chemdraw Professional 16.0 software (PerkinElmer Informatics, Waltham, MA, USA).
3.3. Synthesis of (E)-5-(Methoxyimino)-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one (4)
3,4-Dihydro-1H-benzo[b]azepin-2,5-dione (3, 7.000 g, 39.96 mmol) and O-methylhydroxylamine (2.0 eq, 3.750 g, 79.69 mmol) were suspended in ethanol (110 mL), and pyridine (1.7 eq, 5.6 mL, 69 mmol) was added. The mixture was refluxed for 4 h, clearing to form a yellow solution. When this was left to cool, a pale yellow precipitate formed. The solid was filtered off in vacuo and washed with small portions of ethanol and water. A colourless solid (11.749 g) was obtained after drying at 60 °C for 3 h. The product was dispersed in water (145 mL) and stored for 3 days in the fridge (4 °C). After vacuum filtration and drying for 4 h at 60 °C, the refined colourless solid remained (5.930 g, 29.04 mmol, 73%).
M.p.: 191–192 °C; IR (KBr): ṽ [cm−1] = 3192 (N-H), 3071, 1685 (C=O/C=N), 1607, 1486, 1399, 1041, 873, 768; 1H-NMR (DMSO-d6, 500 MHz): δ (ppm) = 2.40–2.46 (m, 2H, azepine-CH2), 2.93–2.99 (m, 2H, azepine-CH2), 3.92 (s, 3H, O-CH3), 7.04 (dd, J = 8.0/1.2 Hz, 1H, ArH), 7.14 (td, J = 7.6/1.2 Hz, 1H, ArH), 7.36–7.43 (m, 1H, ArH), 7.46 (dd, J = 7.8/1.6 Hz, 1H, ArH), 9.77 (s, 1H, NH); 13C-NMR (DMSO-d6, 126 MHz): δ (ppm) = 61.8, (CH3), 28.6, 30.6 (CH2), 121.9, 124.2, 129.4, 130.3 (CH), 127.3, 137.8, 157.3, 172.9 (C); C11H12N2O2 (204.23); CHN: calc.: C 64.69, H 5.92, N 13.72, found: C 64.92, H 5.80, N 13.54; APCI-MS (ASAP, positive): m/z (relative intensity %): 205 [M + H]+ (48), 173 [M-31]+ (100); HPLC (gradient, AUC %): 99.6% at 254 nm, tMS = 8.0 min, tM (DMSO) = 1.2 min; HPLC (isocratic): 99.6% at 254 nm, tMS = 3.7 min, tM (DMSO) = 1.2 min (ACN/water 30:70); λmax [nm] = 223. Refer to Supplementary Materials for original IR, 1H-NMR, 13C-NMR and mass spectra.
3.4. Crystal Growing and X-ray Structure Determination of (E)-5-(Methoxyimino)-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one (4)
A filtered solution (β(4) = 5 mg/mL) of the compound in acetonitrile was transferred into a small test tube. This was placed in a larger vial containing an atmosphere saturated with petroleum ether. The mixture was stored in the dark at room temperature and periodically checked for crystal formation. Crystals formed after 25 days.
A colourless tablet ca. 0.2 × 0.2 × 0.1 mm was mounted on a nylon loop. Intensity data were recorded at 100 K on a Rigaku/Oxford XtaLAB Synergy diffractometer using a HyPix detector. Crystal data: Monoclinic, space group P21/n, a = 4.15379(16), b = 27.5755(10), c = 8.8092(3) Å, β = 101.842°, V = 987.56 Å3, Z = 4, Dx = 1.374 Mg m−3, F(000) = 432. Data collection and reduction: A total of 70,900 intensities were recorded to 2θmax 70° (98.5% complete to 69°). Of these, 4190 were independent (Rint 0.035). Absorption corrections and frame scaling were based on multi-scans. Structure refinement: The structure was refined anisotropically on F2 using the program SHELXL-2017 [15]. The NH hydrogen atom was refined freely. The methyl was refined as an idealised rigid group allowed to rotate but not tip. All other hydrogens were included using a riding model starting from calculated positions. The final wR2 value was 0.119 (R1 0.040 for reflections > 4σ(F)) for 140 parameters; S 1.04, max./min. ∆ρ 0.48/−0.22 e Å−3.
Supplementary Materials
The following are available online. IR, 1H-NMR, 13C-NMR and mass spectra. CCDC Deposition number 2048455 contains the complete supplementary crystallographic data. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Author Contributions
Conceptualisation, C.K.; methodology, I.I. and P.G.J.; formal analysis, I.I. and P.G.J.; investigation, I.I. and P.G.J.; data curation, I.I. and P.G.J.; writing—original draft preparation, C.K., I.I. and P.G.J.; writing—review and editing, C.K. and P.G.J.; supervision, C.K.; project administration, C.K.; funding acquisition, C.K. All authors have read and agreed to the published version of the manuscript.
Funding
I.I. and C.K. gratefully acknowledge the support by Deutsche Forschungsgemeinschaft, DFG research grant number KU 1371/9-1. We acknowledge support by the Open Access Publication Funds of Technische Universität Braunschweig.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and in the Supplementary Materials.
Acknowledgments
I.I. is thankful for NMR spectra acquisition and processing by the NMR spectroscopy facility, Institut für Organische Chemie, Technische Universität Braunschweig, Hagenring 30, 38106 Braunschweig, Germany (head: Kerstin Ibrom). Furthermore, I.I. is thankful for NMR sample preparation, elemental analysis and acquisition of FT-IR spectra by S. Meyer and P. Reich, Institut für Medizinische und Pharmazeutische Chemie, Technische Universität Braunschweig, Beethovenstraße 55, 38106 Braunschweig, Germany.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Leost, M.; Schultz, C.; Link, A.; Wu, Y.Z.; Biernat, J.; Mandelkow, E.M.; Bibb, J.A.; Snyder, G.L.; Greengard, P.; Zaharevitz, D.W.; et al. Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur. J. Biochem. 2000, 267, 5983–5994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, C.; Link, A.; Leost, M.; Zaharevitz, D.W.; Gussio, R.; Sausville, E.A.; Meijer, L.; Kunick, C. Paullones, a series of cyclin-dependent kinase inhibitors: Synthesis, evaluation of CDK1/cyclin B inhibition, and in vitro antitumor activity. J. Med. Chem. 1999, 42, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Tolle, N.; Kunick, C. Paullones as inhibitors of protein kinases. Curr. Top. Med. Chem. 2011, 11, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Ryczak, J.; Papini, M.; Lader, A.; Nasereddin, A.; Kopelyanskiy, D.; Preu, L.; Jaffe, C.L.; Kunick, C. 2-Arylpaullones are selective antitrypanosomal agents. Eur. J. Med. Chem. 2013, 64, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Mesías, A.C.; Sasoni, N.; Arias, D.G.; Pérez Brandán, C.; Orban, O.C.F.; Kunick, C.; Robello, C.; Comini, M.A.; Garg, N.J.; Zago, M.P. Trypanothione synthetase confers growth, survival advantage and resistance to anti-protozoal drugs in Trypanosoma cruzi. Free Radic. Biol. Med. 2019, 130, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.; Benítez, D.; Korn, R.S.; Ferreira, V.C.; Barrera, E.; Carrión, F.; Pritsch, O.; Pantano, S.; Kunick, C.; de Oliveira, C.I.; et al. Mechanistic and biological characterisation of novel N5-substituted paullones targeting the biosynthesis of trypanothione in Leishmania. J. Enzym. Inhib. Med. Chem. 2020, 35, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Maiwald, F.; Benítez, D.; Charquero, D.; Dar, M.A.; Erdmann, H.; Preu, L.; Koch, O.; Hölscher, C.; Loaëc, N.; Meijer, L.; et al. 9- and 11-Substituted 4-azapaullones are potent and selective inhibitors of African trypanosoma. Eur. J. Med. Chem. 2014, 83, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Kupetz, E.; Preu, L.; Kunick, C.; Bunjes, H. Parenteral formulation of an antileishmanial drug candidate--tackling poor solubility, chemical instability, and polymorphism. Eur. J. Pharm. Biopharm. 2013, 85, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Benítez, D.; Medeiros, A.; Fiestas, L.; Panozzo-Zenere, E.A.; Maiwald, F.; Prousis, K.C.; Roussaki, M.; Calogeropoulou, T.; Detsi, A.; Jaeger, T.; et al. Identification of novel chemical scaffolds inhibiting trypanothione synthetase from pathogenic trypanosomatids. PLoS Negl. Trop. Dis. 2016, 10, e0004617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orban, O.C.F.; Korn, R.S.; Benítez, D.; Medeiros, A.; Preu, L.; Loaëc, N.; Meijer, L.; Koch, O.; Comini, M.A.; Kunick, C. 5-Substituted 3-chlorokenpaullone derivatives are potent inhibitors of Trypanosoma brucei bloodstream forms. Bioorganic Med. Chem. 2016, 24, 3790–3800. [Google Scholar] [CrossRef] [PubMed]
- Mirjafary, Z.; Abdoli, M.; Saeidian, H.; Kakanejadifard, A.; Farnia, S.M.F. Review of the synthesis of acyclic and cyclic oxime ethers. RSC Adv. 2016, 6, 17740–17758. [Google Scholar] [CrossRef]
- Todd, W.M. Cefpodoxime proxetil: A comprehensive review. Int. J. Antimicrob. Agents 1994, 4, 37–62. [Google Scholar] [CrossRef]
- Kunick, C. Synthese [b]-kondensierter Azepindione durch Dealkoxycarbonylierung. Arch. Pharm. Pharm. Med. Chem. 1991, 324, 579–581. [Google Scholar] [CrossRef]
- Learmonth, D.A.; Benes, J.; Parada, A.; Hainzl, D.; Beliaev, A.; Bonifácio, M.J.; Matias, P.M.; Carrondo, M.A.; Garrett, J.; Soares-da-Silva, P. Synthesis, anticonvulsant properties and pharmacokinetic profile of novel 10,11-dihydro-10-oxo-5H-dibenz[b,f]azepine-5-carboxamide derivatives. Eur. J. Med. Chem. 2001, 36, 227–236. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).