Ambient-Temperature Synthesis of (E)-N-(3-(tert-Butyl)-1-methyl-1H-pyrazol-5-yl)-1-(pyridin-2-yl)methanimine
Abstract
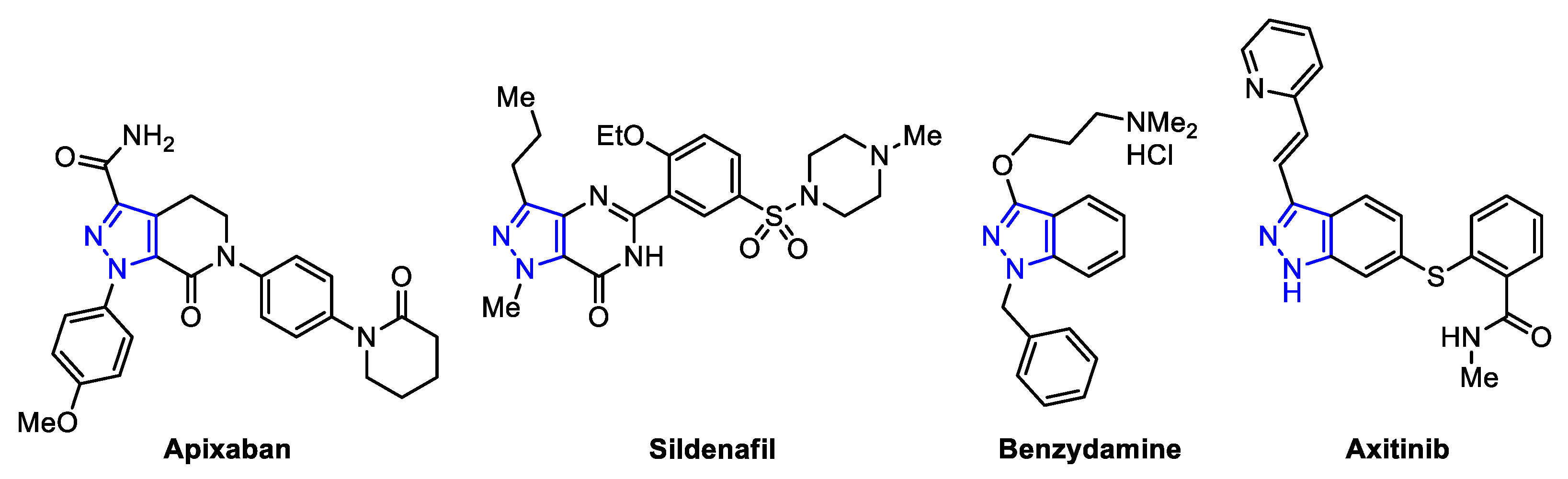
:1. Introduction
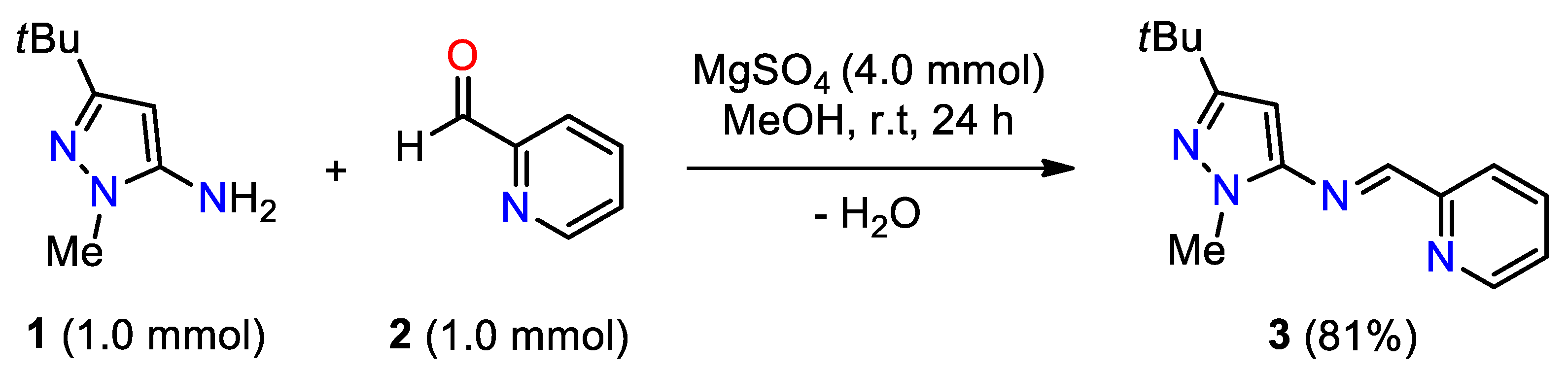
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of (E)-N-(3-(tert-Butyl)-1-methyl-1H-pyrazol-5-yl)-1-(pyridin-2-yl)methanamine 3
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Castillo, J.-C.; Portilla, J. Recent advances in the synthesis of new pyrazole derivatives. Targets Heterocycl. Syst. 2018, 22, 194–223. [Google Scholar] [CrossRef]
- Insuasty, B.; Ramírez, J.; Becerra, D.; Echeverry, C.; Quiroga, J.; Abonia, R.; Robledo, S.M.; Vélez, I.D.; Upegui, Y.; Muñoz, J.A.; et al. An efficient synthesis of new caffeine-based chalcones, pyrazolines and pyrazolo [3,4-b][1,4]diazepines as potential antimalarial, antitrypanosomal and antileishmanial agents. Eur. J. Med. Chem. 2015, 93, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman, S. Review: Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M. Synthesis and pharmacological activities of pyrazole derivatives: A review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.; Faouzi, M.E.A. Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorg. Chem. 2020, 97, 103470. [Google Scholar] [CrossRef] [PubMed]
- Afsina, C.M.A.; Aneeja, T.; Neetha, M.; Anilkumar, G. Recent advances in the synthesis of pyrazole derivatives. Curr. Org. Synth. 2021, 18, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, Y.; Tu, Z. Pyrazole scaffold synthesis, functionalization, and applications in Alzheimer’s disease and Parkinson’s disease treatment (2011–2020). Molecules 2021, 26, 1202. [Google Scholar] [CrossRef] [PubMed]
- Boolell, M.; Allen, M.J.; Ballard, S.A.; Gepi-Attee, S.; Muirhead, G.J.; Naylor, A.M.; Osterloh, I.H.; Gingell, C. Sildenafil: An orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int. J. Impot. Res. 1996, 8, 47–52. [Google Scholar] [PubMed]
- Baldock, G.A.; Brodie, R.R.; Chasseaud, L.F.; Taylor, T.; Walmsley, L.M.; Catanese, B. Pharmacokinetics of benzydamine after intravenous, oral, and topical doses to human subjects. Biopharm. Drug Dispos. 1991, 12, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Michaelson, M.D.; Redman, B.G.; Hudes, G.R.; Wilding, G.; Figlin, R.A.; Ginsberg, M.S.; Kim, S.T.; Baum, C.M.; DePrimo, S.E.; et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2006, 24, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Kumar, V.; Kumar, R.; Singh, S.P. Approaches towards the synthesis of 5-aminopyrazoles. Beilstein J. Org. Chem. 2011, 7, 179–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias-Gómez, A.; Godoy, A.; Portilla, J. Functional pyrazolo[1,5-a]pyrimidines: Current approaches in synthetic transformations and uses as an antitumor scaffold. Molecules 2021, 26, 2708. [Google Scholar] [CrossRef] [PubMed]
- Orrego-Hernández, J.; Lizarazo, C.; Cobo, J.; Portilla, J. Pyrazolo-fused 4-azafluorenones as key reagents for the synthesis of fluorescent dicyanovinylidene-substituted derivatives. RSC Adv. 2019, 9, 27318–27323. [Google Scholar] [CrossRef] [Green Version]
- Galvez, J.; Castillo, J.-C.; Quiroga, J.; Rajzmann, M.; Rodriguez, J.; Coquerel, Y. Divergent chemo-, regio-, and diastereoselective normal electron-demand Povarov-type reactions with α-oxo-ketene dienophiles. Org. Lett. 2014, 16, 4126–4129. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.-C.; Quiroga, J.; Abonia, R.; Rodriguez, J.; Coquerel, Y. The aryne aza-Diels–Alder reaction: Flexible syntheses of isoquinolines. Org. Lett. 2015, 17, 3374–3377. [Google Scholar] [CrossRef] [PubMed]
- Becerra, D.; Rojas, H.; Castillo, J.-C. 3-(tert-Butyl)-N-(4-methoxybenzyl)-1-methyl-1H-pyrazol-5-amine. Molbank 2021, 2021, M1196. [Google Scholar] [CrossRef]
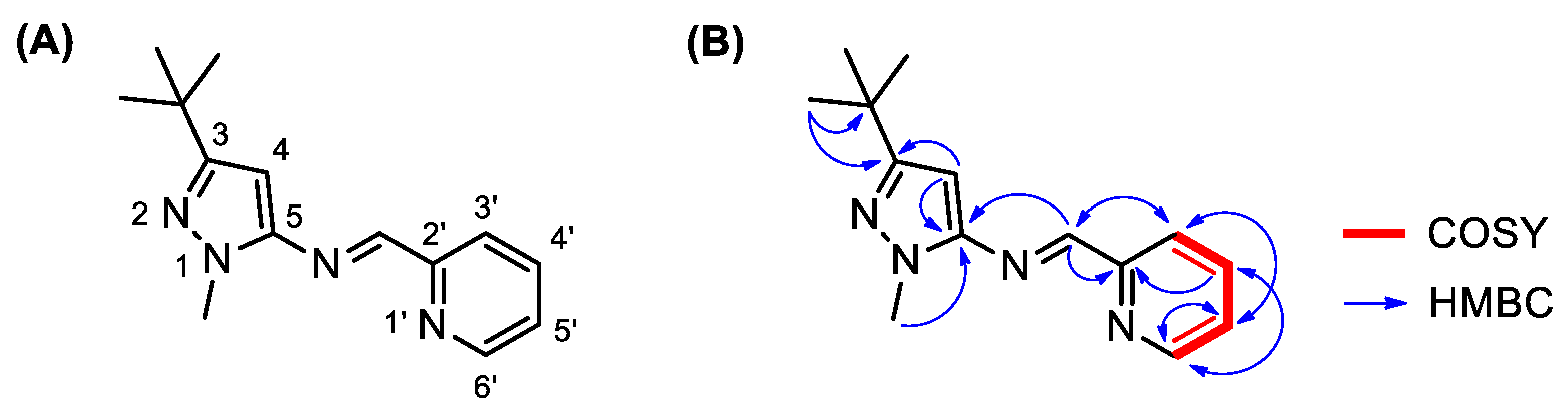
| Number | δH (Mult, J in Hz) | δC (ppm) | COSY (1H-1H) | HMBC (1H-13C) |
|---|---|---|---|---|
| CH3, t-Bu | 1.32 (s) | 30.6 | -- | -- |
| Cq, t-Bu | -- | 32.4 | -- | CH3, t-Bu (2J) |
| NCH3 | 3.94 (s) | 34.8 | -- | -- |
| 3 | -- | 161.4 | -- | H-4 (2J) CH3, t-Bu (3J) |
| 4 | 6.17 (s) | 88.7 | -- | -- |
| 5 | -- | 148.9 | -- | CH=N (3J) H-4 (2J) NCH3 (3J) |
| 2′ | -- | 154.7 | -- | CH=N (2J) H-4′ (3J) |
| 3′ | 8.21 (dt, J = 7.6, 1.0) | 121.5 | H-4′ (3J) | CH=N (3J) H-5′ (3J) |
| 4′ | 7.78 (dddd, J = 7.8, 7.6, 1.6, 0.8) | 136.7 | H-3′ (3J) H-5′ (3J) | H-6′ (3J) |
| 5′ | 7.34 (ddd, J = 7.6, 4.8, 1.2) | 125.3 | H-4′ (3J) H-6′ (3J) | H-6′ (2J) H-3′ (3J) |
| 6′ | 8.68 (ddd, J = 4.8, 1.6, 0.8) | 149.9 | H-5′ (3J) | H-4′ (3J) H-5′ (2J) |
| CH=N | 8.66 (s) | 159.1 | -- | H-3′ (3J) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerra, D.; Cobo, J.; Castillo, J.-C. Ambient-Temperature Synthesis of (E)-N-(3-(tert-Butyl)-1-methyl-1H-pyrazol-5-yl)-1-(pyridin-2-yl)methanimine. Molbank 2021, 2021, M1250. https://doi.org/10.3390/M1250
Becerra D, Cobo J, Castillo J-C. Ambient-Temperature Synthesis of (E)-N-(3-(tert-Butyl)-1-methyl-1H-pyrazol-5-yl)-1-(pyridin-2-yl)methanimine. Molbank. 2021; 2021(3):M1250. https://doi.org/10.3390/M1250
Chicago/Turabian StyleBecerra, Diana, Justo Cobo, and Juan-Carlos Castillo. 2021. "Ambient-Temperature Synthesis of (E)-N-(3-(tert-Butyl)-1-methyl-1H-pyrazol-5-yl)-1-(pyridin-2-yl)methanimine" Molbank 2021, no. 3: M1250. https://doi.org/10.3390/M1250
APA StyleBecerra, D., Cobo, J., & Castillo, J.-C. (2021). Ambient-Temperature Synthesis of (E)-N-(3-(tert-Butyl)-1-methyl-1H-pyrazol-5-yl)-1-(pyridin-2-yl)methanimine. Molbank, 2021(3), M1250. https://doi.org/10.3390/M1250






