Synthesis and Characterization of Novel Thiazolidinones and Thioxothiazolidinones Derived from Substituted Indole
Abstract
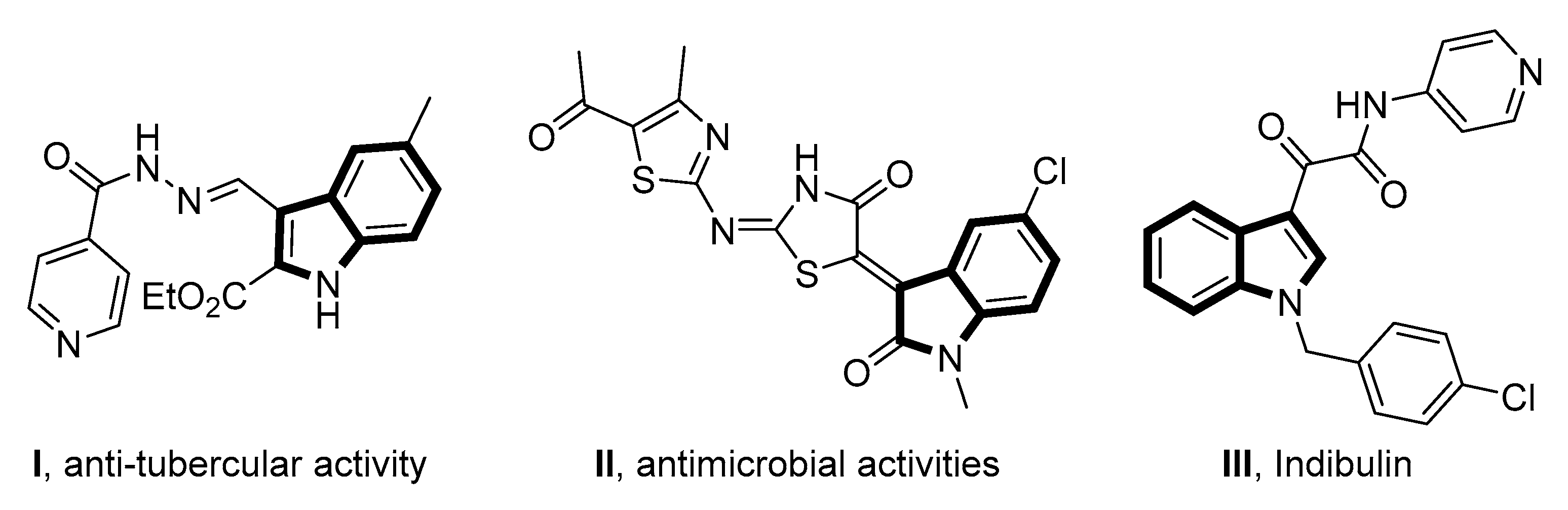
:1. Introduction
2. Results and Discussion
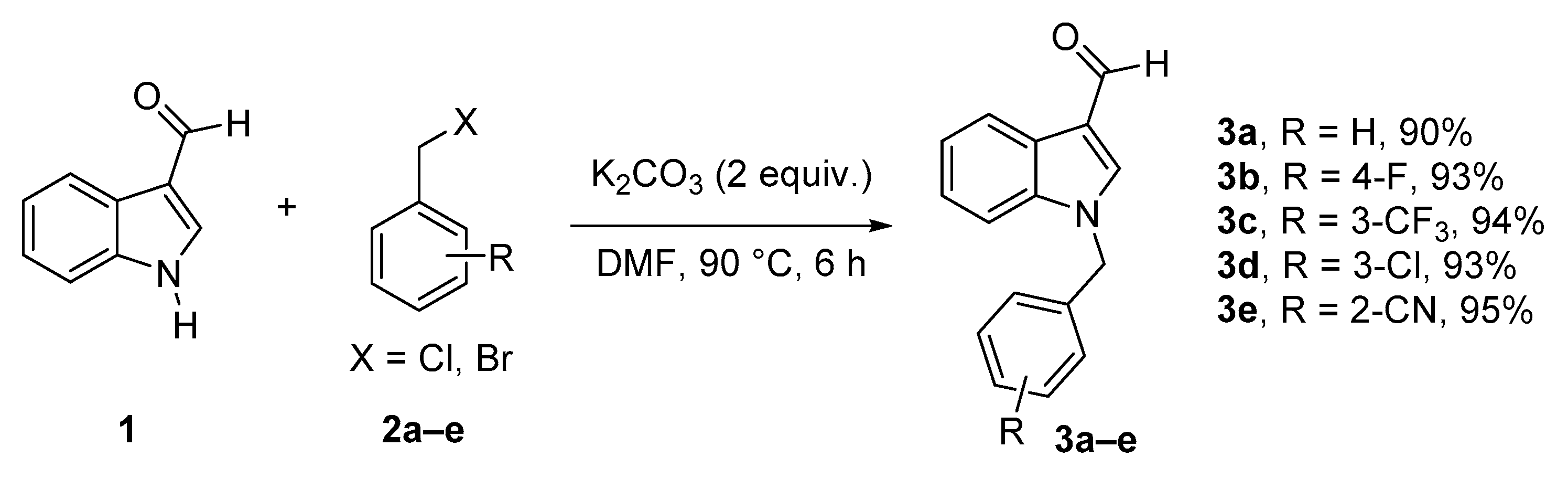
2.1. Synthesis of N-Benzylindole-3-carboaldehyde Derivatives
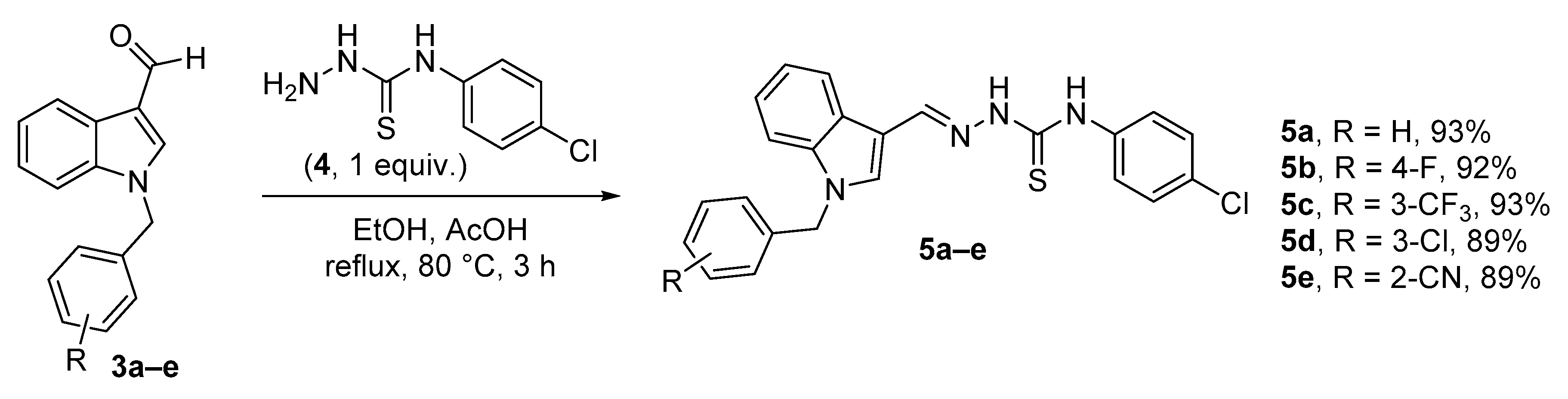
2.2. Synthesis of Thiosemicarbazones
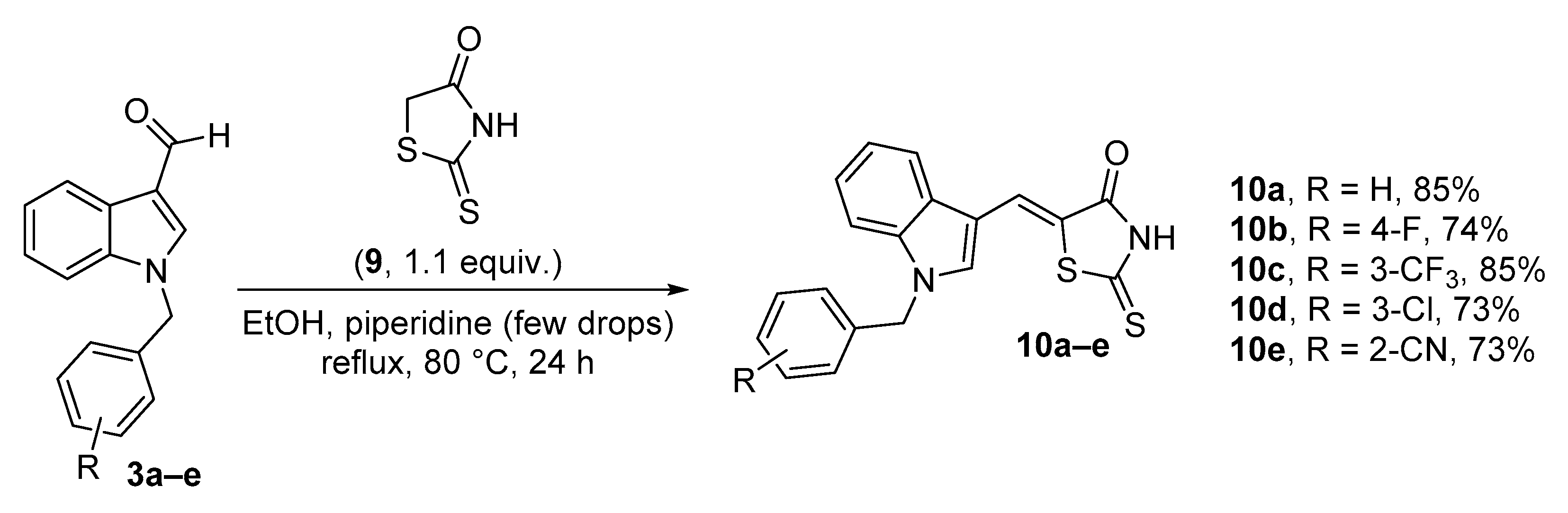
2.3. Synthesis of Thiazolidin-4-One Derivatives
3. Materials and Methods
3.1. General Information
3.2. Experimental Section
3.2.1. General Procedure for the Synthesis of 1-Substituted-1H-indole-3-carbaldehydes (3a–e) (35)
3.2.2. General Procedure for the Synthesis of Thiosemicarbazones (5a–e) (35)
3.2.3. General Procedure for the Synthesis of Thiazolidin-4-ones (7a–e)
3.2.4. General Procedure for the Synthesis of Thioxothiazolinones (10a–e)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Verma, P.; Yadav, B.; Komath, S.S. Synthesis and evaluation of indole-based new scaffolds for antimicrobial activities-identification of promising candidate. Bioorg. Med. Chem. Lett. 2011, 21, 3367–3372. [Google Scholar] [CrossRef] [PubMed]
- Minvielle, M.J.; Eguren, K.; Melander, C. Highly active modulators of indole signaling alter pathogenic behaviors in gram-negative and gram-positive bacteria. Chem. Eur. J. 2013, 19, 17595–17602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Mulholland, N.; Beattie, D.; Irwin, D.; Gu, Y.C.; Chen, Q.; Yang, G.F.; Clough, J. Synthesis and antifungal activity of 3-(1,3,4-oxadiazol-5-yl)-indoles and 3-(1,3,4-oxadiazol-5-yl)methyl-indoles. Eur. J. Med. Chem. 2013, 63, 22–32. [Google Scholar] [CrossRef]
- Mandour, A.H.; El-Sawy, E.R.; Shaker, K.H.; Mustafa, M.A. Synthesis, anti-inflammatory, analgesic and anticonvulsant activities of 1,8-dihydro-1-aryl-8-alkyl pyrazolo(3,4-b)indoles. Acta Pharm. 2010, 60, 73–88. [Google Scholar] [CrossRef] [Green Version]
- Guerra, A.S.; Malta, D.J.; Laranjeira, L.P.; Maia, M.B.; Colaco, N.C.; de Lima Mdo, C.; Galdino, S.L.; Pitta Ida, R.; Goncalves-Silva, T. Anti-inflammatory and antinociceptive activities of indole-imidazolidine derivatives. Int. Immunopharmacol. 2011, 11, 1816–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdemir, A.; Altintop, M.D.; Turan-Zitouni, G.; Ciftci, G.A.; Ertorun, I.; Alatasc, O.; Kaplancikli, Z.A. Synthesis and evaluation of new indole-based chalcones as potential antiinflammatory agents. Eur. J. Med. Chem. 2015, 89, 304–309. [Google Scholar] [CrossRef]
- Gitto, R.; Luca, L.D.; Ferro, S.; Citraro, R.; Sarro, G.D.; Costa, L.; Ciranna, L.; Chimirri, A. Development of 3-substituted-1H-indole derivatives as NR2B/NMDA receptor antagonists. Bioorg. Med. Chem. 2009, 17, 1640–1647. [Google Scholar] [CrossRef]
- Huang, S.-M.; Hsu, P.-C.; Chen, M.-Y.; Li, W.-S.; More, S.V.; Lu, K.-T.; Wang, Y.-C. The novel indole compound SK228 induces apoptosis and FAK/Paxillin disruption in tumor cell lines and inhibits growth of tumor graft in the nude mouse. Int. J. Cancer 2012, 131, 722–732. [Google Scholar] [CrossRef]
- Ahn, S.; Hwang, D.J.; Barrett, C.M.; Yang, J.; Duke, C.B., III; Miller, D.D.; Dalton, J.T. A novel bis-indole destabilizes microtubules and displays potent in vitro and in vivo antitumor activity in prostate cancer. Cancer Chemoth. Pharmacol. 2011, 67, 293–304. [Google Scholar] [CrossRef]
- Schuck, D.C.; Jordao, A.K.; Nakabashi, M.; Cunha, A.C.; Ferreira, V.F.; Garcia, C.R.S. Synthetic indole and melatonin derivatives exhibit antimalarial activity on the cell cycle of the human malaria parasite Plasmodium falciparum. Eur. J. Med. Chem. 2014, 78, 375–382. [Google Scholar] [CrossRef]
- Nagalakshmi, G.; Maity, T.K.; Maiti, B.C. Synthesis, characterization and antiviral evaluation of some novel 2-[(substitutedphenyl/heteroaryl)imino]-3-phenyl-1,3-thiazolidin-4-ones. Der Pharm. Lett. 2013, 5, 177–188. [Google Scholar]
- Patil, A.P.; Patel, T.K.; Patil, A.R.; Patil, C.S.; Patil, S.T.; Pawar, P. Chemistry and biological activity of 4-thiazolidinone. World J. Pharm. Pharm. Sci. 2015, 4, 1780–1791. [Google Scholar]
- Wang, S.; Zhao, Y.; Zhang, G.; Lv, Y.; Zhang, N.; Gong, P. Design, synthesis and biological evaluation of novel 4-thiazolidinones containing indolin-2-one moiety as potential antitumor agent. Eur. J. Med. Chem. 2011, 46, 3509–3518. [Google Scholar] [CrossRef]
- Monte, C.D.; Carradori, S.; Bizzarri, B.; Bolasco, A.; Caprara, F.; Mollica, A.; Rivanera, D.; Mari, E.; Zicari, A.; Akdemir, A. Anti-Candida activity and cytotoxicity of a large library of new N-substituted-1,3-thiazolidin-4-one derivatives. Eur. J. Med. Chem. 2016, 107, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Abo-Ashour, M.F.; Eldehna, W.M.; George, R.F.; Abdel-Aziz, M.M.; Elaasser, M.M.; Gawad, N.M.A.; Gupta, A.; Bhakta, S.; Abou-Seri, S.M. Novel indole thiazolidinone conjugates: Design, synthesis and whole-cell phenotypic evaluation as a novel class of antimicrobial agents. Eur. J. Med. Chem. 2018, 160, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottana, R.; Maccari, R.; Giglio, M.; Del Corso, A.; Cappiello, M.; Mura, U.; Cosconati, S.; Marinelli, L.; Novellino, E.; Sartini, S.; et al. Identification of 5-arylidene-4 thiazolidinone derivatives endowed with dual activity as aldose reductase inhibitors and antioxidant agents for the treatment of diabetic complications. Eur. J. Med. Chem. 2011, 46, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Ishak, E.A.; El Malah, T.; Brown, A.B.; Elayat, W.M. Synthesis of potentially antioxidant and antibacterial biologically active thiazolidines. J. Heterocycl. Chem. 2015, 52, 1758–1764. [Google Scholar] [CrossRef]
- Rawal, R.K.; Tripathi, R.; Katti, S.B.; Pannecouque, C.; de Clercq, E. Design, synthesis, and evaluation of 2-aryl-3-heteroaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Bioorg. Med. Chem. 2007, 15, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Masic, L.P.; Tomasic, T. Rhodanine as a privileged scaffold in drug discovery. Curr. Med. Chem. 2009, 16, 1596–1629. [Google Scholar]
- Nitsche, C.; Klein, C.D. Aqueous microwave-assisted one-pot synthesis of N-substituted rhodanines. Tetrahedron Lett. 2012, 53, 5197–5201. [Google Scholar] [CrossRef]
- Yarovenko, V.N.; Nikitina, A.S.; Zavarzin, I.V.; Krayushkin, M.M.; Kovalenko, L.V. A convenient synthesis of N-substituted 2-thioxo-1,3-thiazolidin-4-ones. Synthesis 2006, 8, 1246–1248. [Google Scholar] [CrossRef]
- Velezheva, V.; Brennan, P.; Ivanov, P.; Kornienko, A.; Lyubimov, S.; Kazarian, K.; Nikonenko, B.; Majorov, K.; Apt, A. Synthesis and antituberculosis activity of indole-pyridine derived hydrazides, hydrazide-hydrazones, and thiosemicarbazones. Bioorg. Med. Chem. 2016, 26, 978–985. [Google Scholar] [CrossRef]
- Bacher, G.; Nickel, B.; Emig, P.; Vanhoefer, U.; Seeber, S.; Shandra, A.; Klenner, T.; Beckers, T. D-24851, a novel stnthetic microytubule inhibitor, exerts curative antitumoral activity in vivo, shows efficacy towrd multidrug-resistant tumor celles and lacks neurotoxicity. Cancer Res. 2001, 61, 392–399. [Google Scholar]
- Benmohammed, A.; Khoumeri, O.; Djafri, A.; Terme, T.; Vanelle, P. Synthesis of novel highly functionalized 4-thiazolidinone derivatives from 4-phenyl-3-thiosemicarbazones. Molecules 2014, 19, 3068–3083. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Bao, G.; Wang, L.; Li, W.; Xu, B.; Du, B.; Lv, J.; Zhai, X.; Gong, P. Synthesis, biological evaluation and preliminary mechanism study of novel benzothiazole derivatives bearing indole-based moiety as potent antitumor agents. Eur. J. Med. Chem. 2015, 96, 173–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sing, W.T.; Lee, C.L.; Yeo, S.L.; Lim, S.P.; Sim, M.M. Arylalkylidene rhodanine with bulky and hydrophobic functional group as selective HCV NS3 protease inhibitor. Bioorg. Med. Chem. Lett. 2001, 11, 91–94. [Google Scholar] [CrossRef]
- Radi, M.; Botta, L.; Casaluce, G.; Bernardini, M.; Botta, M. Practical One-Pot Two-Step Protocol for the Microwave-Assisted Synthesis of Highly Functionalized Rhodanine Derivatives. J. Comb. Chem. 2010, 12, 200–205. [Google Scholar] [CrossRef]
- Kumar, B.R.P.; Soni, M.; Kumar, S.S.; Singh, K.; Patil, M.; Baig, R.B.N.; Adhikary, L. Synthesis, glucose uptake activity and structure–activity relationships of some novel glitazones incorporated with glycine, aromatic and alicyclic amine moieties via two carbon acyl linker. Eur. J. Med. Chem. 2011, 46, 835–844. [Google Scholar] [CrossRef]
- Kumar, B.R.P.; Nanjan, M.J. Novel glitazones: Design, synthesis, glucose uptake and structure–activity relationships. Bioorg. Med. Chem. Lett. 2010, 20, 1953–1956. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.R.P.; Nanjan, M.J. QSAR Study on Thiazolidine-2,4-dione Derivatives for Antihyperglycemic Activity. Indian J. Pharm. Sci. 2008, 70, 565–571. [Google Scholar]
- Kumar, B.R.P.; Desai, B.J.; Vergheese, J.; Praveen, T.K.; Suresh, B.; Nanjan, M.J. CoMFA Study on Thiazolidine-2,4-diones for their Antihyperglycemic Activity. Lett. Drug Des. Discov. 2008, 5, 79–87. [Google Scholar]
- Kumar, B.R.P.; Karvekar, M.D.; Adhikary, L.; Nanjan, M.J.; Suresh, B. Microwave induced synthesis of the thiazolidine-2,4-dione motif and the efficient solvent free-solid phase parallel syntheses of 5-benzylidene-thiazolidine-2,4-dione and 5-benzylidene-2-thioxo-thiazolidine-4-one compounds. J. Heterocycl. Chem. 2006, 45, 897–903. [Google Scholar] [CrossRef]
- Kumar, B.R.P.; Praveen, T.K.; Nanjan, M.J.; Karvekar, M.D.; Suresh, B. Serum glucose and triglyceride lowering activity of some novel glitazones against dexamethasone-induced hyperlipidemia and insulin resistance. Indian J. Pharmacol. 2007, 39, 299–302. [Google Scholar]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Benmohammed, A.; Rebika, N.; Sehanine, Y.; Louail, A.E.; Khoumeri, O.; Kadiri, M.; Djafri, A.; Terme, T.; Vanelle, P. Synthesis and antimicrobial activities of new thiosemicarbazones and thiazolidinones in indole series. Monatsh. Chem. 2021, 152, 977–986. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rekiba, N.; Benmohammed, A.; Khanoussi, S.; Djafri, A.; Thibonnet, J. Synthesis and Characterization of Novel Thiazolidinones and Thioxothiazolidinones Derived from Substituted Indole. Molbank 2021, 2021, M1284. https://doi.org/10.3390/M1284
Rekiba N, Benmohammed A, Khanoussi S, Djafri A, Thibonnet J. Synthesis and Characterization of Novel Thiazolidinones and Thioxothiazolidinones Derived from Substituted Indole. Molbank. 2021; 2021(4):M1284. https://doi.org/10.3390/M1284
Chicago/Turabian StyleRekiba, Nawel, Abdelmadjid Benmohammed, Sofiane Khanoussi, Ayada Djafri, and Jérôme Thibonnet. 2021. "Synthesis and Characterization of Novel Thiazolidinones and Thioxothiazolidinones Derived from Substituted Indole" Molbank 2021, no. 4: M1284. https://doi.org/10.3390/M1284
APA StyleRekiba, N., Benmohammed, A., Khanoussi, S., Djafri, A., & Thibonnet, J. (2021). Synthesis and Characterization of Novel Thiazolidinones and Thioxothiazolidinones Derived from Substituted Indole. Molbank, 2021(4), M1284. https://doi.org/10.3390/M1284






