Abstract
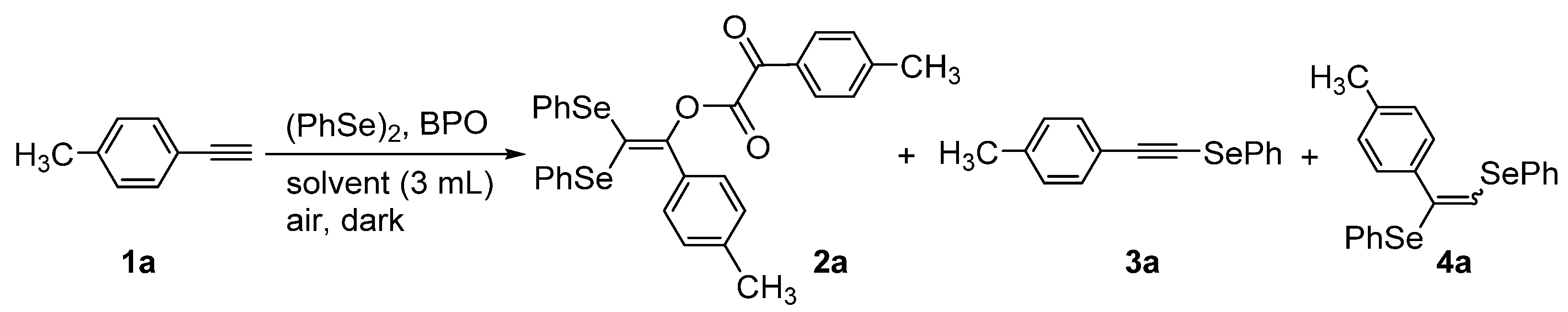
2,2-Bis(phenylselanyl)-1-(p-tolyl)vinyl 2-oxo-2-(p-tolyl)acetate was synthesized via the reaction of p-tolylacetylene with diphenyl diselenide and benzoyl peroxide in benzene under atmospheric conditions. The molecular structure of the synthesized compound was evaluated using single-crystal X-ray analysis and spectral analyses. The process reported here provides a rare example of the direct and selective transformation of a terminal alkyne to the corresponding geminal diseleno-substituted alkene.
1. Introduction
Atomically efficient and selective addition reaction of heteroatom compounds to carbon−carbon unsaturated bonds is one of the most valuable synthetic reactions [1,2,3,4,5,6,7,8,9,10,11,12]. The addition of heteroatom compounds to alkynes affords the corresponding alkenyl heteroatoms, which are typically present not only in synthetic intermediates but also in a variety of natural products and functional molecules [13,14,15,16,17,18].
During the course of our study on the simultaneous introduction of different heteroatom groups into alkynes [19,20,21,22,23,24,25,26], we successfully developed a binary system composed of benzoyl peroxide [(PhCOO)2; BPO] and diphenyl diselenide [(PhSe)2] for the selective selenation of alkynes [26]. Specifically, in the case of internal alkynes, the stereoselective benzoyloxyselenation reaction yielded the corresponding β-(benzyloxy)alkenyl selenides, whereas terminal alkynes were converted to alkynyl selenides via a C(sp)–H substitution reaction with (PhSe)2 (Scheme 1). Herein, we report a unique multi-coupling reaction of a terminal alkyne (p-tolylacetylene, 1a), BPO, and (PhSe)2 to produce a novel 1,2-diketone compound (2a) containing a geminal diseleno-substituted alkene moiety (see, Scheme 2).
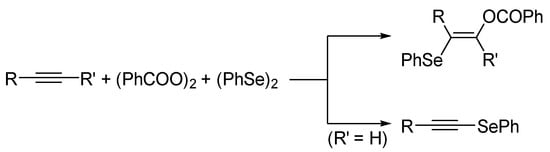
Scheme 1.
Selective selenation of alkynes based on a BPO/(PhSe)2 binary system.
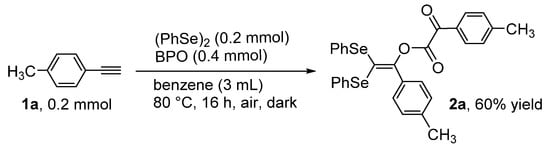
Scheme 2.
Synthesis of 2a through the reaction of 1a with (PhSe)2 and BPO.
2. Results and Discussion
During the reaction of p-tolylacetylene (1a) with (PhSe)2 (1 equiv) and BPO (2 equiv) in benzene, the title coupling product, 2a, was formed in 60% yield. The structure of this product was determined using X-ray diffraction (Scheme 2 and Figure 1) and several spectral analyses (the 1H, 13C{1H} and 77Se{1H} NMR spectra of 2a are included in the Supplementary Materials). As indicated in Scheme 2, the molecular structure of 2a consists of one molecule of (PhSe)2 and two molecules of the terminal alkyne 1a.
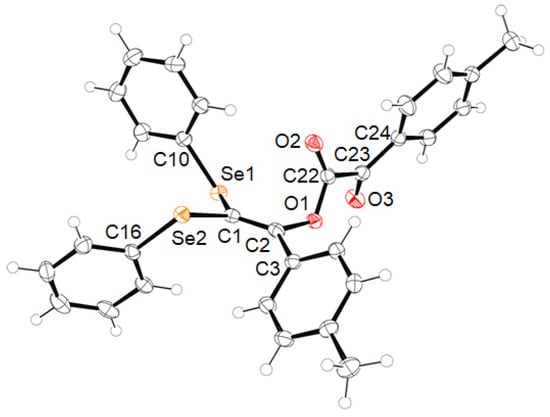
Figure 1.
Crystal structure of 2a with numbered atoms. Ellipsoids are shown at the 50% probability level. Selected interatomic distances (Å) and angles (deg): Se1−C1, 1.9178(17); Se1−C10, 1.916(2); Se2−C1, 1.9021(19); Se2−C16, 1.9188(19); O1−C2, 1.420(2); O1−C22, 1.360(2); O2−C22, 1.195(2); O3−C23, 1.215(2); C1−C2, 1.338(3); C2−C3, 1.482(3); C22−C23, 1.546(3); C23−C24, 1.478(3); C1−Se1−C10, 99.28(8); C1−Se2−C16, 101.23(8); C2−O1−C22, 116.88(14); Se1−C1−Se2, 120.61(10); Se1−C1−C2, 120.87(14); Se2−C1−C2, 118.38(13); O1−C2−C1, 119.30(16); O1−C2−C3, 111.41(15); C1−C2−C3, 129.04(17); O1−C22−O2, 125.80(17); O1−C22−C23, 109.00(15); O2−C22−C23, 125.14(17); O3−C23−C22, 117.29(16); O3−C23−C24, 123.59(17); C22−C23−C24, 119.09(16).
The multi-coupling reaction of 1a, BPO, and (PhSe)2 was further evaluated under various reaction conditions (Table 1). Increasing the amount of (PhSe)2 did not induce a significant increase in the yield of 2a, whereas byproducts 3a and 4a were formed (entry 1 vs. entry 2). Prolonging the reaction time was also ineffective in improving the yield of 2a (entry 1 vs. entry 3). When the amount of (PhSe)2 was reduced to 0.10 mmol and an excess amount (0.60 mmol) of BPO was introduced, 3a was formed as the primary reaction product (entry 4). Among the solvents used, benzene produced the most encouraging results (entry 1 vs. entries 5−7). The yield of 2a was slightly improved by reducing the amount of the benzene solvent used (entry 8). When the reaction was conducted using concentrated benzene solvent (heated at 80 °C without cooling water), the yield of 2a was improved to 63% (entry 9). However, a further increase in the reaction temperature to 100 °C decreased the yield of 2a (entries 10 and 11).

Table 1.
Optimization of the reaction conditions for the synthesis of 2a.
To evaluate the formation mechanism of 2a, the reaction of alkynyl selenide 3a was attempted using equimolar amounts of (PhSe)2 and BPO to produce the coupling product 2a in a 61% yield (Scheme 3). The result suggests that alkynyl selenide 3a acts as an intermediate during the formation of 2a. It should be noted that 2a possesses an α-keto ester moiety, which may be formed during the oxidation of the alkynyl ether moiety using molecular oxygen (O2). Wille et al. previously reported the oxidation of alkynes (e.g., PhC≡CPh) to produce 1,2-diketones (e.g., PhC(=O)–C(=O)Ph) in the presence of O2, in a process initiated by the thiyl radical (PhS•). More specifically, the thiylperoxyl radical (PhSOO•), derived from PhS• and O2, acted as an initiator during the oxidation reaction [27]. Therefore, we assume that the C≡C triple bond in the alkynyl ether intermediate 6a (or 4a), a precursor to 2a, underwent oxidation with O2 in the presence of the selenoperoxyl radical PhSeOO•, which acts as an initiator. Although this process has several potential reaction routes, one of the most promising is given in Scheme 4. The thermal decomposition of BPO generates a benzoyloxy radical, which attacks 3a to produce alkynyl benzoate, 4a. The subsequent electrophilic reaction of 3a with 4a in the presence of 5a produced 6a along with benzoic acid anhydride. Notably, this reaction provides a rare example of the direct and selective transformation of a terminal alkyne to the corresponding geminal diseleno-substituted alkene [28,29,30].
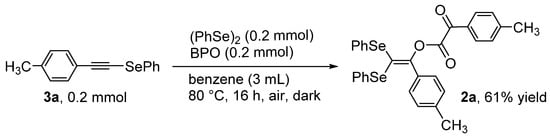
Scheme 3.
Synthesis of 2a via the reaction of 3a with (PhSe)2 and BPO.
Scheme 4.
Possible pathway for the multi-coupling reaction.
3. Experimental
3.1. General
1H-NMR spectra were obtained using a JEOL JMN-ECX400 (400 MHz) FT NMR system (JEOL, Tokyo, Japan), in which CDCl3 was employed as the deuterated solvent and Me4Si was used as an internal standard. 13C{1H} NMR spectra were obtained using a JEOL JNM-ECX400 (100 MHz) FT NMR system (JEOL, Tokyo, Japan). The 77Se{1H} NMR spectrum was obtained using a JEOL JNM-ECX400 (76 MHz) FT NMR system (JEOL, Tokyo, Japan). Unless otherwise stated, all reagents and solvents were purchased from chemical companies and used without further purification.
3.2. Synthesis of 2,2-Bis(phenylselanyl)-1-(p-tolyl)Vinyl 2-Oxo-2-(p-tolyl)Acetate (2a)
Initially, BPO (0.20 mmol), (PhSe)2 (0.20 mmol), p-tolylacetylene (1a) (0.20 mmol), and benzene (3 mL) were added to a 10 mL round-bottomed flask. The resultant mixture was then heated at 80 °C for 16 h in the dark, under atmospheric conditions. Once the reaction was complete, the reaction mixture was treated with saturated aqueous sodium thiosulfate. The product was then extracted using ethyl acetate, and the resultant combined organic layer was washed using saturated aqueous sodium bicarbonate. The organic layer was neutralized with aqueous HCl (0.1 N). Subsequently, the combined extracts were dried using MgSO4. Filtration and concentration in vacuo yielded the crude product, which was subsequently purified using GPC (eluent CHCl3) to obtain 2a (35.4 mg, 60% yield) as a pale-yellow solid. Mp 65.0–66.0 °C; 1H-NMR (CDCl3) δ 7.97 (d, J = 8.2 Hz, 2H), 7.52 (d, J = 7.8 Hz, 2H), 7.37 (dd, J = 8.5, 1.1 Hz, 2H), 7.27–7.17 (m, 12H), 2.41 (s, 3H), 2.36 (s, 3H); 13C{1H} NMR (CDCl3) δ 184.4, 160.9, 150.8, 146.4, 139.9, 134.8, 132.3, 131.4, 131.1, 130.5, 130.0, 129.9, 129.6, 129.2, 128.81, 128.77, 128.7, 128.0, 127.3, 113.7, 21.9, 21.5; 77Se{1H} NMR (CDCl3) δ 452, 431; IR (KBr, cm−1): 2360, 2331, 1748, 1683, 1604, 1577, 1475, 1438, 1151, 954, 735; MS (FAB): m/z = 592 [M]+. HRMS (ESI) analysis of 2a also showed m/z 468.9585 attributed to the sodium ion adduct of (PhSe)2CHC(=O)(C6H4-p-CH3) (calcd m/z: 468.9586) generated via decomposition of the thermally unstable 1,2-diketone moiety of 2a during the analysis, which supports the identification of 2a.
3.3. X-ray Diffraction Studies
An X-ray crystallographic measurement was carried out on a Rigaku RAXIS-RAPID diffractometer with Mo-Kα radiation at 123 K. Of 39479 reflections collected, 5743 were unique (Rint = 0.0465). An empirical absorption correction was applied, which resulted in transmission factors ranging from 0.513 to 0.942. The data were corrected for Lorentz and polarization effects. The structure of 2a was solved by direct methods and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically, and hydrogen atoms were refined using the riding model. All calculations were performed with the CrystalStructure [31] crystallographic software package except for refinements, which was performed using SHELXL Version 2014/7 [32].
Crystallographic data: formula weight = 590.44; monoclinic; space group P21/n; a = 9.62321(17) Å, b = 5.73648(10) Å, c = 45.1254(8) Å; V = 2490.50(8) Å3; Z = 4; ρcalcd = 1.575 g cm−3; total reflections collected = 39479; GOF = 1.054; R1 = 0.0264; wR2 = 0.0672. Crystallographic data have been deposited with Cambridge Crystallographic Data Centre (CCDC-2104238). These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed date: 19 August 2021) (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
4. Conclusions
A unique multi-coupling reaction of the terminal alkyne 1a, (PhSe)2, and BPO was observed, the mechanism of which was based on an oxidation process. We believe that the results presented here provide a novel and facile route for the synthesis of geminal diseleno-substituted alkenes.
Supplementary Materials
The following are available: Figure S1. 1H-NMR spectrum (CDCl3, 400 MHz) of compound 2a. Figure S2. 13C{1H} NMR spectrum (CDCl3, 100 MHz) of compound 2a. Figure S3. 77Se{1H} NMR spectrum (CDCl3, 76 MHz) of compound 2a.
Author Contributions
Investigation, S.K., V.T.H., T.S., K.M., Y.Y., M.S., A.N. and A.O.; Experiment, T.S., K.M. and Y.Y.; resources, S.K., A.N. and A.O.; writing—original draft preparation, S.K.; writing—review and editing, S.K., Y.Y., A.N. and A.O.; funding acquisition, S.K., A.N. and A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by JSPS KAKENHI Grant Numbers JP21H01977, JP19H02791, and JP19H02756, from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and in its Supplementary Materials.
Acknowledgments
A part of this work was conducted in NAIST, supported by Nanotechnology Platform Program (Synthesis of Molecules and Materials) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beletskaya, I.; Moberg, C. Element−Element Addition to Alkynes Catalyzed by the Group 10 Metals. Chem. Rev. 1999, 99, 3435–3461. [Google Scholar] [CrossRef]
- Han, L.-B.; Tanaka, M. Transition metal-catalysed addition reactions of H-heteroatom and inter-heteroatom bonds to carbon−carbon unsaturated linkages via oxidative additions. Chem. Commun. 1999, 5, 395–402. [Google Scholar] [CrossRef]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Transition-Metal-Catalyzed Addition of Heteroatom−Hydrogen Bonds to Alkynes. Chem. Rev. 2004, 104, 3079–3160. [Google Scholar] [CrossRef]
- Beletskaya, I.; Moberg, C. Element−Element Additions to Unsaturated Carbon−Carbon Bonds Catalyzed by Transition Metal Complexes. Chem. Rev. 2006, 106, 2320–2354. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Ananikov, V.P. Transition-Metal-Catalyzed C−S, C−Se, and C−Te Bond Formation via Cross-Coupling and Atom-Economic Addition Reactions. Chem. Rev. 2011, 111, 1596–1636. [Google Scholar] [CrossRef] [PubMed]
- Ansell, M.B.; Navarro, O.; Spencer, J. Transition metal catalyzed element-element’ additions to alkynes. Coord. Chem. Rev. 2017, 336, 54–77. [Google Scholar] [CrossRef]
- Kawaguchi, S.-I.; Yamamoto, Y.; Ogawa, A. Catalytic synthesis of sulfur and phosphorus compounds via atom-economic reactions. Mendeleev Commun. 2020, 30, 129–138. [Google Scholar] [CrossRef]
- Chiummiento, L.; D’Orsi, R.; Funicello, M.; Lupattelli, P. Last Decade of Unconventional Methodologies for the Synthesis of Substituted Benzofurans. Molecules 2020, 25, 2327. [Google Scholar] [CrossRef]
- Li, G.L.; Huo, X.H.; Jiang, X.Y.; Zhang, W.B. Asymmetric synthesis of allylic compounds via hydrofunctionalisation and difunctionalisation of dienes, allenes, and alkynes. Chem. Soc. Rev. 2020, 49, 2060–2118. [Google Scholar] [CrossRef]
- Miyaura, N. Catalytic Heterofunctionalization; Togni, A., Grutzmacher, H., Eds.; Wiley-VCH: Weinheim, Germany, 2001; pp. 1–32. [Google Scholar]
- Brunet, J.J.; Neibecker, D. Catalytic Heterofunctionalization; Togni, A., Grutzmacher, H., Eds.; Wiley-VCH: Weinheim, Germany, 2001; pp. 91–141. [Google Scholar]
- Ogawa, A. Transition-Metal-Catalyzed S−H and Se−H to Unsaturated Molecules. Top. Organomet. Chem. 2013, 43, 325–360. [Google Scholar]
- Coronado, E.; Forment-Aliaga, A.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J.; Martinéz-Ferrero, E.; Nuez, A.; Romero, F.M. Multifunctional molecular materials. Solid State Sci. 2003, 5, 917–924. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ghosh, S.; Ghosh, M. The thio-Claisen rearrangement 1980–2001. Tetrahedron 2003, 59, 7251–7271. [Google Scholar] [CrossRef]
- Martín Castro, A.M. Claisen Rearrangement over the Past Nine Decades. Chem. Rev. 2004, 104, 2939–3002. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, E.A.; Stivala, C.A.; Zakarian, A. [3,3]-Sigmatropic rearrangements: Recent applications in the total synthesis of natural products. Chem. Soc. Rev. 2009, 38, 3133–3148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millemaggi, A.; Taylor, R.J.K. 3-Alkenyl-oxindoles: Natural products, pharmaceuticals, and recent synthetic advances in tandem/telescoped approaches. Eur. J. Org. Chem. 2010, 2010, 4527–4547. [Google Scholar] [CrossRef]
- Palomba, M.; Franco Coelho Dias, I.; Rosati, O.; Marini, F. Modern Synthetic Strategies with Organoselenium Reagents: A Focus on Vinyl Selenones. Molecules 2021, 26, 3148. [Google Scholar] [CrossRef]
- Ogawa, A. Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4, pp. 392–441. [Google Scholar]
- Nomoto, A.; Ogawa, A. Preparative Uses of Organoselenium and Organotellurium Compounds. In The Chemistry of Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; Wiley: Chichester, UK, 2012; Volume 3, pp. 623–688. [Google Scholar]
- Tsuchii, K.; Imura, M.; Kamada, N.; Hirao, T.; Ogawa, A. An Efficient Photoinduced Iodoperfluoroalkylation of Carbon−Carbon Unsaturated Compounds with Perfluoroalkyl Iodides. J. Org. Chem. 2004, 69, 6658–6665. [Google Scholar] [CrossRef]
- Kawaguchi, S.-I.; Shirai, T.; Ohe, T.; Nomoto, A.; Sonoda, M.; Ogawa, A. Highly Regioselective Simultaneous Introduction of Phosphino and Seleno Groups into Unsaturated Bonds by the Novel Combination of (Ph2P)2 and (PhSe)2 upon Photoirradiation. J. Org. Chem. 2009, 74, 1751–1754. [Google Scholar] [CrossRef]
- Tamai, T.; Nomoto, A.; Tsuchii, K.; Minamida, Y.; Mitamura, T.; Sonoda, M.; Ogawa, A. Highly selective perfluoroalkylchalcogenation of alkynes by the combination of iodoperfluoroalkanes and organic dichalcogenides upon photoirradiation. Tetrahedron 2012, 68, 10516–10522. [Google Scholar] [CrossRef]
- Kawaguchi, S.-I.; Ogawa, A. Highly selective addition of phosphorus-containing interelement compounds to alkynes. Synlett 2013, 24, 2199–2215. [Google Scholar]
- Yoshimura, A.; Takamachi, Y.; Han, L.B.; Ogawa, A. Organosulfide-Catalyzed Diboration of Terminal Alkynes under Light. Chem.-Eur. J. 2015, 21, 13930–13933. [Google Scholar] [CrossRef]
- Kodama, S.; Saeki, T.; Mihara, K.; Higashimae, S.; Kawaguchi, S.-I.; Sonoda, M.; Nomoto, A.; Ogawa, A. A Benzoyl Peroxide/Diphenyl Diselenide Binary System for Functionalization of Alkynes Leading to Alkenyl and Alkynyl Selenides. J. Org. Chem. 2017, 82, 12477–12484. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.J.; Wille, U. Activation of Molecular Oxygen by SRadicals: Experimental and Computational Studies on a Novel Oxidation of Alkynes to α-Diketones. Chem. Commun. 2008, 46, 6239–6241. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, I.; Henriksen, L.; Eggert, H. 77Se NMR. 2. The Basis for Application of JSe–Se and JSe–H in Structure Assignments of Mono-, Di-, and Triseleno-Substituted Alkenes. J. Org. Chem. 1986, 51, 1657–1663. [Google Scholar] [CrossRef]
- Moro, A.V.; Nogueira, C.W.; Barbosa, N.B.V.; Menezes, P.H.; Rocha, J.B.T.; Zeni, G. Highly Stereoselective One-Pot Procedure To Prepare Bis- and Tris-chalcogenide Alkenes via Addition of Disulfides and Diselenides to Terminal Alkynes. J. Org. Chem. 2005, 70, 5257–5268. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.C.; Godoi, B.; Prigol, M.; Nogueira, C.W.; Zeni, G. Highly Stereoselective One-Pot Procedure to Prepare Unsymmetrical Bis- and Tris-chalcogenide Alkenes via Addition of Chalcogens to Alkynes. Organometallics 2007, 26, 4252–4256. [Google Scholar] [CrossRef]
- CrystalStructure 4.2: Crystal Structure Analysis Package; Rigaku Corporation: Tokyo, Japan, 2000–2015.
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).