tert-Butyl Bis(4′-(Hexyloxy)-[1,1′-biphenyl]-4-yl)carbamate
Abstract
:1. Introduction
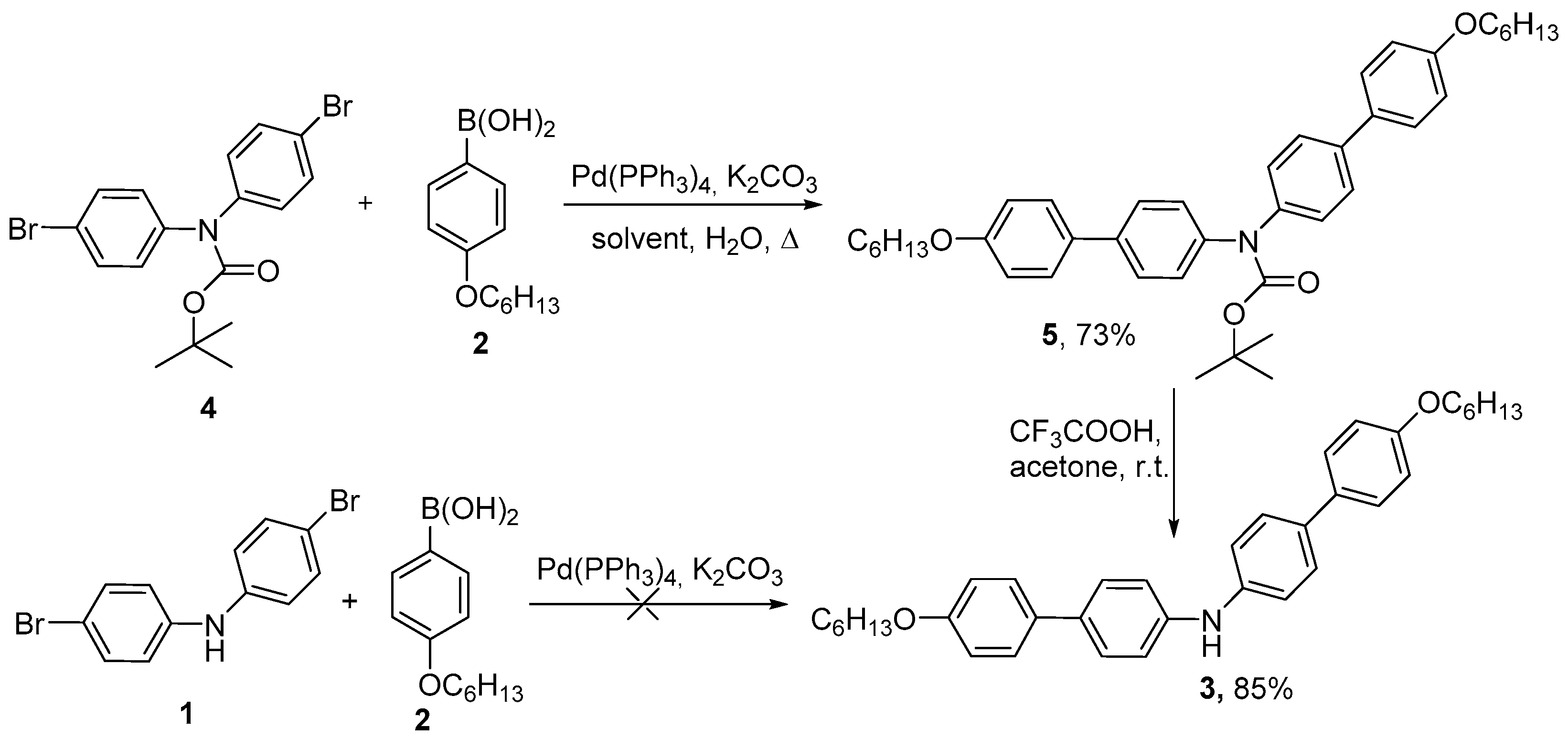
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baheti, A.; Singh, P.; Lee, C.-P.; Thomas, K.R.J.; Ho, K.-C. 2,7-Diaminofluorene-based organic dyes for dye-sensitized solar cells: Effect of auxiliary donor on optical and electrochemical properties. J. Org. Chem. 2011, 76, 4910–4920. [Google Scholar] [CrossRef] [PubMed]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Lee, C.-P.; Li, C.-T.; Ho, K.-C. Use of organic materials in dye-sensitized solar cells. Mater. Today 2017, 20, 267–283. [Google Scholar] [CrossRef]
- Carella, A.; Borbone, F.; Centore, R. Research Progress on Photosensitizers for DSSC. Front. Chem. 2018, 6, 481. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, M.S.; Gudim, N.S.; Knyazeva, E.A.; Tanaka, E.; Zhang, L.; Mikhalchenko, L.V.; Robertson, N.; Rakitin, O.A. 9-(p-Tolyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole—A new donor building-block in the design of sensitizers for dye-sensitized solar cells. J. Photochem. Photobiol. A 2020, 391, 112333. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Chmovzh, T.N.; Golovanov, I.S.; Knyazeva, E.A.; Mikhalchenko, L.V.; Saifutyarov, R.S.; Avetisov, I.C.; Woollins, J.D.; Taydakov, I.V.; Rakitin, O.A. Candle light-style OLEDs with benzochalcogenadiazoles cores. Dyes Pigm. 2021, 185, 108917. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Mikhailov, M.S.; Chmovzh, T.N.; Vaschenko, A.A.; Gudim, N.S.; Mikhalchenko, L.V.; Taydakov, I.V.; Rakitin, O.A. A novel D-A-D fluorescent dyes based on 9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole as a donor unit for solution-processed organic light-emitting-diodes. Molecules 2021, 26, 2872. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ren, Y.; Zhang, W.; Wu, Y.; Socie, E.C.; Carlsen, B.I.; Moser, J.-E.; Tian, H.; Zakeeruddin, S.M.; Zhu, W.-H.; et al. Phenanthrene-fused-quinoxaline as a key building block for highly efficient and stable sensitizers in copper-electrolyte-based dye-sensitized solar cells. Angew. Chem. Int. Ed. 2020, 59, 9324–9329. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.D.; Choi, I.T.; Kim, H.K. D–p–A organic dyes with various bulky amine-typed donor moieties for dye-sensitized solar cells employing the cobalt electrolyte. Org. Electron. 2015, 25, 1–5. [Google Scholar] [CrossRef]
- Bonn, A.G.; Neuburger, M.; Wenger, O.S. Photoinduced electron transfer in rhenium(i)-oligotriarylamine molecules. Inorg. Chem. 2014, 53, 11075–11085. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Su, L.; Bo, Z. Hyperbranched polymers with a degree of branching of 100% prepared by catalyst transfer suzuki-miyaura polycondensation. J. Am. Chem. Soc. 2009, 131, 10348–10349. [Google Scholar] [CrossRef] [PubMed]
| Entry | Solvent | Temperature, °C | Time, h | Yield, of 5% |
|---|---|---|---|---|
| 1 | THF | 78 | 24 | 15 |
| 2 | Dioxane | 81 | 50 | 65 |
| 3 | Toluene | 110 | 50 | 73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmovzh, T.N.; Rakitin, O.A. tert-Butyl Bis(4′-(Hexyloxy)-[1,1′-biphenyl]-4-yl)carbamate. Molbank 2021, 2021, M1247. https://doi.org/10.3390/M1247
Chmovzh TN, Rakitin OA. tert-Butyl Bis(4′-(Hexyloxy)-[1,1′-biphenyl]-4-yl)carbamate. Molbank. 2021; 2021(3):M1247. https://doi.org/10.3390/M1247
Chicago/Turabian StyleChmovzh, Timofey N., and Oleg A. Rakitin. 2021. "tert-Butyl Bis(4′-(Hexyloxy)-[1,1′-biphenyl]-4-yl)carbamate" Molbank 2021, no. 3: M1247. https://doi.org/10.3390/M1247
APA StyleChmovzh, T. N., & Rakitin, O. A. (2021). tert-Butyl Bis(4′-(Hexyloxy)-[1,1′-biphenyl]-4-yl)carbamate. Molbank, 2021(3), M1247. https://doi.org/10.3390/M1247







