Abstract
(PyH)2[MoOCl5] was obtained in the form of emerald green crystals unintentionally from (PyH)5[MoOCl4(H2O)]3Cl2 in acetonitrile. (PyH)2[MoOCl5] has been used as a starting material in molybdenum(V) coordination chemistry for decades, yet its true identity has not been known until now. The X-ray structure analysis has undoubtedly confirmed the existence of this compound. The [MoOCl5]2− ion displays the usual structural characteristics of the mononuclear MoO3+-containing compounds.
1. Introduction
The main motivation for pursuing research on the coordination chemistry of molybdenum has been its biological importance. As of today, molybdenum has been found in over 50 enzymes [1]. Two groups may be recognised: nitrogenases with an iron–molybdenum cluster as a cofactor and a larger group of enzymes with a pterin-based cofactor [1]. These enzymes catalyse oxidation-reduction reactions that form part of nitrogen, sulphur, and carbon metabolism [2]. Versatile redox behaviour and oxophilic nature make molybdenum suitable for this role. Molybdenum has a strong tendency to bind oxygen and, at the same time, the capacity to lose it. In the course of catalysis, molybdenum shuttles between oxidation states +6, +5, and +4 with some reactions involving oxygen atom transfer (OAT) processes.
We have been interested in the coordination chemistry of the intermediate oxidation state, +5 [3,4]. The MoO3+ structural entity pervades this oxidation state. Owing to its multiple bond character, it lies at the origin of the geometric distortions of molybdenum(V) complex species which are eventually manifested also in their reactivity. Herein, a crystal structure of (PyH)2[MoOCl5], another MoO3+-containing species, will be presented. The compounds containing [MoOCl5]2− ions were prepared as early as 1927 [5]. (PyH)2[MoOCl5] formed upon the molybdate(VI) reduction with hydrazine in 11 M hydrochloric acid. After the addition of pyridine, needle-shaped crystals of emerald green colour formed [6]. The compound was of interest as it provided, in spite of its inherent air sensitivity, a suitable entry into the molybdenum(V) coordination chemistry. A drawback of using this compound was that its true identity was not known until 2005 [7]. Its composition was proposed on the basis of the elemental analyses on Cl and Mo. On the other hand, a comparison of the reflectance spectrum of the solid ammonium pentachloridooxomolybdate(V) with the spectrum of its HCl solution has suggested the presence of either the [MoOCl5]2− or the [MoOCl4(H2O)]− ions [8]. The X-ray structure analysis of crystals obtained by a modified procedure, a reduction of MoO3 with hydroiodic acid in concentrated HCl(aq), followed by the addition of pyridine, has disclosed the [MoOCl4(H2O)]− ions as the only molybdenum(V) species in the product. The correct composition of the emerald green crystals, unequivocally established by the X-ray structure analysis, turned out to be more complex than initially assumed. The [MoOCl4(H2O)]− ions co-crystallised with chloride and pyridinium cations resulting in the (PyH)5[MoOCl4(H2O)]3Cl2 formula [7]. It is of interest to note that (PyH)5[MoOCl4(H2O)]3Cl2 and the at-first-proposed (PyH)2[MoOCl5] share similar Cl and Mo contents. The chlorine and the molybdenum contents in (PyH)5[MoOCl4(H2O)]3Cl2 are 38.57% and 22.37%, whereas for (PyH)2[MoOCl5], the values amount to 39.44% and 21.35%, respectively. The “missing” compound, (PyH)2[MoOCl5], has been obtained just recently in our laboratory. It was isolated in the course of the (PyH)5[MoOCl4(H2O)]3Cl2 reactions with pyrazinecarboxylic acid in acetonitrile. With this reaction aiming towards the coordination of pyrazinecarboxylic acid to molybdenum(V), the formation of (PyH)2[MoOCl5] was surprising. Although the reaction was found to be reproducible, no rational explanation can be provided for the transformation of [MoOCl4(H2O)]− into [MoOCl5]2−. The starting material, (PyH)5[MoOCl4(H2O)]3Cl2, which is the source of chloride, provides only two-thirds of the amount necessary for a quantitative transformation. Even more puzzling is the role of pyrazinecarboxylic acid. In its absence, no (PyH)2[MoOCl5] could be isolated. Although the synthetic conditions necessary for the formation of (PyH)2[MoOCl5] remain as elusive as ever, our study undoubtedly confirms its existence. We may also conclude that with the available literature data, it remains unclear whether the early reports were on (PyH)5[MoOCl4(H2O)]3Cl2 or (PyH)2[MoOCl5].
2. Results
The solid-state structure of pyridinium pentachloridooxomolybdate(V) consists of the mononuclear [MoOCl5]2− anions and protonated pyridine molecules as countercations. Although part of the [MoOCl5]2− ion resides on the twofold rotation axis, its symmetry is that of the C4v point group. The drawing of the formula unit is shown in Figure 1, and relevant geometric parameters of the [MoOCl5]2− ion are listed in Table 1.
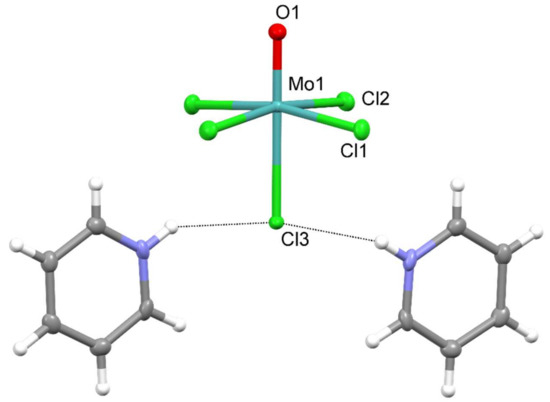
Figure 1.
Displacement ellipsoids plot of formula unit in (PyH)2[MoOCl5]. The ellipsoids are drawn at a 50% probability level, whereas hydrogen atoms are drawn as spheres of arbitrary radii. Dotted lines are the N–H∙∙∙Cl interactions.

Table 1.
Relevant geometric parameters [Å,°] for the [MoOCl5]2− ion of (PyH)2[MoOCl5].
The most prominent feature of the complex ion is the Mo=O structural fragment with the Mo–O length of 1.665(3) Å. The six-coordinate metal environment consists of the oxide and five chlorides. The equatorial chlorides, Cl(1), Cl(2), and their symmetry counterparts, are at 2.3845(8)–2.4072(8) Å, whilst Cl(3), which occupies the position trans to the multiply bonded oxide, is at a significantly longer distance, 2.6038(10) Å. The highly distorted octahedral environment of molybdenum(V) is a result of an operating trans influence of the multiply bonded oxide [9]. With the metal ion being located 0.2333(7) Å above the best plane of four equatorial chlorides, the shape of the [MoOCl5]2− ion may be described as umbrella-like.
It should be noted that the lengthening of the Mo(1)–Cl(3) bond is a joint result of the operating trans influence of the molybdenyl moiety and the engagement of the Cl(3) chloride in hydrogen bonding with pyridinium cations. Cl(3) forms two hydrogen bonds with two pyridinium cations, with the N(1)∙∙∙Cl(3) contacts being 3.092(3) Å, a significantly shorter distance than the sum of the N and Cl van der Waals radii, 3.3 Å [10]. Such connectivity is known as a bifurcated hydrogen bond. The pattern, the [MoOCl5]2− ion with two hydrogen-bonded pyridinium cations, is shown in Figure 1. These hydrogen-bonded clusters interact with adjacent ones via π∙∙∙π stacking interactions occurring between pairs of pyridinium cations (Table 2, Figure 2 and Figure 3).

Table 2.
Intermolecular interactions [Å, °] in (PyH)2[MoOCl5].
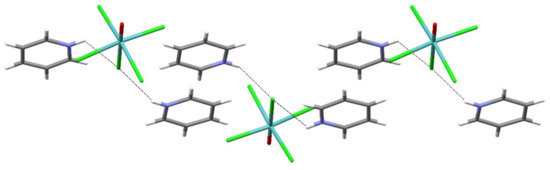
Figure 2.
π∙∙∙π stacking of pyridinium cations in (PyH)2[MoOCl5]. Dotted lines are the N–H∙∙∙Cl hydrogen bonds.
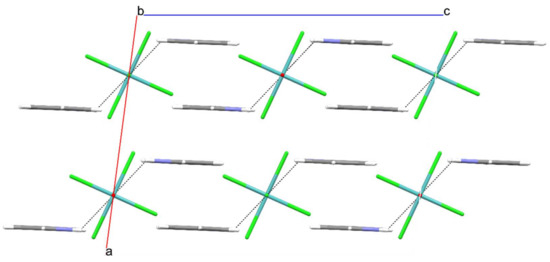
Figure 3.
Packing of ions in (PyH)2[MoOCl5]: a view along b-axis.
There are only six structurally characterised compounds with pentachloridooxomolybdate(V) ions: {HP(Ph)3}2[MoOCl5] [12], (Me4N)2[MoOCl5]∙CH3CN [13], (C5H10NO)2[MoOCl5] (C5H10NO+ = O-protonated valerolactam) [14], (PyH)10[(Mo2O4Cl4)2(µ4-hda)][MoOCl5]Cl2 (where hda2− stands for a dianion of heptanedioic acid) [15], (PyH)2[MoOCl5]∙CH2Cl2 [16], and {P(Ph)4}2[MoOCl5]∙2CH2Cl2 [17]. The listed compounds were products of very diverse reactions, and therefore, no synthetic guidelines concerning the formation of the [MoOCl5]2− ion can be elucidated. The geometry of the [MoOCl5]2− ion in the title compound is very similar to those in the cited examples. A solvate with dichloromethane, (PyH)2[MoOCl5]∙CH2Cl2 [16], features a slightly longer bond between molybdenum and chloride that is trans to the terminal oxide, 2.6910(6) Å. An even larger discrepancy in the molybdenum-to-the-chloride bonding pattern was observed for (C5H10NO)2[MoOCl5] in which the bonds to equatorial chlorides are in the 2.3741(7)–2.3820(7) Å range, whereas the bond to the apical chloride is as long as 2.7582(7) Å [14]. The elongation of this bond was explained with the engagement of the trans-positioned chloride in hydrogen-bonding interactions with the countercations.
3. Materials and Methods
3.1. General
All reagents but (PyH)5[MoOCl4(H2O)]3Cl2 were purchased from commercial sources and used without further purification. Molybdenum(V) starting material was prepared following the published procedure [7]. IR spectrum of the Nujol suspension was recorded in the 4000–600 cm−1 spectral region using an FTIR instrument PerkinElmer Spectrum 100 (PerkinElmer, Shelton, CT, USA). Owing to the decomposition of crystalline (PyH)2[MoOCl5] when exposed to the air atmosphere, no elemental CHN analysis was performed. Single crystal X-ray diffraction data were collected on an Agilent SuperNova diffractometer (Agilent Technologies XRD Products, Oxfordshire, UK) with copper (Cu-Kα, λ = 1.54184 Å) X-ray source at 150 K. CrysAlis PRO [18] was used for data processing and Olex2 software [19] for data analysis. The structure was solved by ShelXT [20] and refined by the least-squares method in ShelXL [21]. Anisotropic displacement parameters were determined for all nonhydrogen atoms. Platon [22] and Mercury [23] were used for the analysis of the crystal structure and the preparation of figures. The crystal structure was deposited to the CCDC and assigned the deposition number 2088930. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk). The crystallographic data are summarised in Table 3.

Table 3.
Crystallographic data for (PyH)2[MoOCl5].
3.2. Synthesis
(PyH)5[MoOCl4(H2O)]3Cl2 (428 mg, 1.00 mmol of molybdenum(V) complex) was dissolved in acetonitrile (20 mL). To thus obtained emerald green solution, pyrazinecarboxylic acid (80 mg, 0.65 mmol) was added. No colour change ensued. The solution was left to stand at ambient conditions in a closed Erlenmeyer flask for an hour. Afterwards, diethyl ether (25 mL) was added dropwise. On the following day, a copious amount of emerald green crystals of (PyH)2[MoOCl5] was obtained. Notes. The crystals decompose almost instantaneously when taken out from the mother liquor. On exposure to the air, the solution acquires a deep violet colour. IR (Nujol, cm−1): 1632, 1601, 1527, 1321, 1236, 1189, 1161, 1080, 1049, 1030, 979, 960 [ν(Mo=O)], 851, 729, 672, 607. The Nujol signature bands are not listed. The IR spectrum may be found in Supplementary Materials.
Supplementary Materials
The following are available online, Figure S1: IR spectrum of (PyH)2[MoOCl5].
Author Contributions
Conceptualisation, J.Š. and B.M.; checking the reproducibility of synthesis, J.Š.; writing—review and editing, J.Š. and B.M. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovenian Research Agency (Program Grant P1-0134).
Informed Consent Statement
Not applicable.
Data Availability Statement
Crystal structure data can be found at CCDC (Deposition Number 2088930).
Acknowledgments
The authors would like to thank Nina Podjed for her help in the manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Burgmayer, S.J.N.; Stiefel, E.I. Molybdenum enzymes, cofactors, and systems: The chemical uniqueness of molybdenum. J. Chem. Educ. 1985, 62, 943. [Google Scholar] [CrossRef] [Green Version]
- Modec, B.; Dolenc, D. Molybdenum complexes with citrate revisited. A mononuclear [MoVOCl4(H2O)]− ion as a new synthetic entry. Inorg. Chim. Acta 2019, 495, 119006. [Google Scholar] [CrossRef]
- Modec, B.; Dolenc, D.; Kasunič, M. Complexation of molybdenum(V) with glycolic acid: An unusual orientation of glycolato ligand in {Mo2O4}2+ complexes. Inorg. Chem. 2008, 47, 3625–3633. [Google Scholar] [CrossRef]
- James, R.G.; Wardlaw, W. Co-ordination compounds of quinquevalent molybdenum. J. Chem. Soc. 1927, 2145–2156. [Google Scholar] [CrossRef]
- Hanson, G.R.; Brunette, A.A.; McDonell, A.C.; Murray, K.S.; Wedd, A.G. Electronic properties of thiolate compounds of oxomolybdenum(V) and their tungsten and selenium analogs. Effects of oxygen-17, molybdenum-98, and molybdenum-95 isotope substitution upon ESR spectra. J. Am. Chem. Soc. 1981, 103, 1953–1959. [Google Scholar] [CrossRef]
- Modec, B.; Brenčič, J.V. Novel methanol-containing oxomolybdate(V) complexes: Synthesis and structural characterisation of intermediates in the formation of {Mo2O4}2+ clusters from [MoOCl4(H2O)]− and [MoOBr4]− precursors. Eur. J. Inorg. Chem. 2005, 2005, 1698–1709. [Google Scholar] [CrossRef]
- Gray, H.B.; Hare, C.R. The electronic structures and spectra of chromyl and molybdenyl ions. Inorg. Chem. 1962, 1, 363–368. [Google Scholar] [CrossRef]
- Blake, A.B.; Cotton, F.A.; Wood, J.S. The crystal, molecular, and electronic structures of a binuclear oxomolybdenum(V) xanthate complex. J. Am. Chem. Soc. 1964, 86, 3024–3031. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Junk, P.C.; Atwood, J.L. Hydrogen-bonded tetramethylethylenediammonium and triphenylphosphonium complexes derived from liquid clathrate media. J. Coord. Chem. 1999, 46, 505–518. [Google Scholar] [CrossRef]
- Seyedsadjadi, S.A.; Ghammamy, S.; Rezaeibehbahani, G. The crystal and molecular structure of bis(tetramethylammonium) pentachlorooxomolybdate(V)-acetonitrile(1:1). Cryst. Res. Technol. 2005, 40, 727–730. [Google Scholar] [CrossRef]
- Marchetti, F.; Pampaloni, G.; Zacchini, S. Lactam/MoCl5 interaction in CH2Cl2: Synthesis and X-ray characterization of protonated δ-valerolactam salts. RSC Adv. 2013, 3, 10007–10013. [Google Scholar] [CrossRef]
- Modec, B.; Brenčič, J.V. Anions of 1,3,5-benzenetricarboxylic and heptanedioic acids serving as bridges between dimolybdenum(V) metal–metal bonded units: Preparation and structural characterization of dinuclear and tetranuclear complexes. Eur. J. Inorg. Chem. 2005, 2005, 4325–4334. [Google Scholar] [CrossRef]
- Vrdoljak, V.; Milić, D.; Cindrić, M.; Matković-Čalogović, D.; Cinčić, D. Synthesis of novel molybdenum(V) complexes: Structural characterization of two thiosemicarbazonato complexes [MoOCl2{C6H4(O)CH:NNHC:SNHC6H5}] and [MoOCl2{C10H6(O)CH:NNHC:SNHC6H5}]·CH3CN, and two oxohalomolybdates NH4[MoOCl4(CH3CN)] and [C5H5NH]2[MoOCl5]·CH2Cl2. Polyhedron 2007, 26, 3363–3372. [Google Scholar] [CrossRef]
- Isovitsch, R.A.; May, J.G.; Fronczek, F.R.; Maverick, A.W. Photoredox reactions of oxomolybdenum(V) with phosphines. Polyhedron 2000, 19, 1437–1446. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, UK, 2014. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).