Abstract
Chloropinane and chloromenthene, synthesized from pinene and limonene, respectively, were compared with their non-halogenated analogs and n-hexane for their ability to solubilize natural products of interest such as β-carotenoids, vanillin, and rosmarinic acid. Chloropinane was six times more efficient than hexane for β-carotene solubilization. Chloromenthene was 15 times better than hexane. Vanillin was 20 times more soluble in chloropinane than in hexane. Chloropinane and chloromenthene were 3.5 and 2 times more efficient than hexane for rosmarinic acid solubilization. Obtained from pinene and limonene, two very abundant natural products, and even from their waste byproducts, chloropinane and chloromenthene can be an alternative to solvents from non-renewable resources.
1. Introduction
Solvents are essential for chemical synthesis, for the extractions of bioactive compounds, and for all separations and purifications of mixtures of compounds. However, most if not the majority of solvents are hydrocarbons derived from fossil resources and are considered hazardous and toxic [1,2]. In a previous study, we showed the potential of pinane as an alternative solvent for the extraction of natural products. Cis-rich pinane (cis/trans: 7/3) solvent was prepared for the first time by a solvent-free catalytic hydrogenation of α/β-pinene or turpentine oil [3,4,5]. Solubilization simulations with Conductor like Screening Model Realistic Solvation (COSMO-RS) software [6] of some natural products have revealed that pinane compares very well with n-hexane and may be an alternative to this fossil-based solvent. When compared experimentally with n-hexane, cis-pinane solubilized 42, 2, and 3 times more of b-carotene, vanillin, and rosmarinic acid, respectively [3,5]. Obtained through limonene’s partial and total hydrogenation under various catalytic conditions, menthene, and menthane were also compared to n-hexane [7]. While COSMO-RS software predicted a comparable solubilization of these β-carotene, vanillin, and rosmarinic acid for the menthene, menthane, and menthene/menthane mixture, experimental assays revealed that menthene solubilizes β-carotene, vanillin, and rosmarinic acid three to five times better than n-hexane [7].
As part of our continuing interest in biobased solvents as potential sustainable and green solvents, we were interested to the halogenated analogs of pinane and menthene for comparison with their non-halogenated analogs and n-hexane. Halogenated solvents may have the advantage of extracting more products due to the greater affinity of the molecules for these solvents. In this study, chloropinane and chloromenthene were synthesized to be compared with pinane, menthene, and n-hexane for the extraction of bioactive compounds. To the best of our knowledge, chloropinane and chloromenthene have never been investigated as solvents for the extraction of natural products.
2. Results and Discussion
2.1. Synthesis
Chloropinane (2) and chloromenthene (4) were synthesized as depicted in Scheme 1 as reported by Beckmann et al. and De Mattos et al. with slight modifications [8,9]. Chloropinane (2) was obtained from β-pinene which was transformed into myrtenol to subsequently be halogenated (Scheme 1). Chloromenthene (4) was obtained by halogenation of limonene (Scheme 1). Cis rich- pinane and menthene were synthesized through a solvent free hydrogenation over Pd/C and Ru/C as reported in our previous contributions [3,4,5,7].
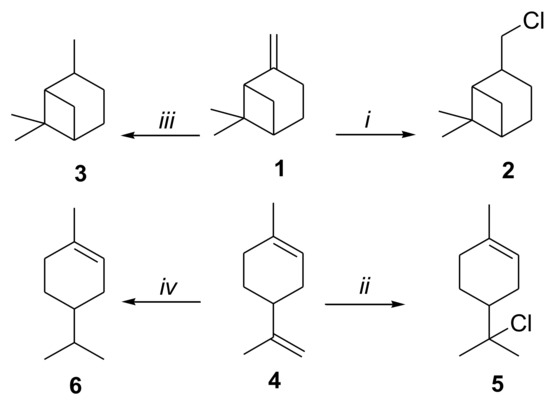
Scheme 1.
(i) (1) NaBH4, Me2SO4, THF, 24 h, rt. (2) NaOH, H2O2, 12 h, rt. (3) PPh3, CCl4. 24 h, reflux; (ii) SOCl2, SiO2, CH2Cl2, 12 h, rt; (iii) Pd/C, 2.75 MPa, rt; (iv) Pd/Al2O3, H2O, 2.75 MPa, rt.
2.2. Prediction of Solubilization with COSMO-RS
The COSMO-RS simulation was conducted to evaluate the potential of pinane, chloropinane (2), cis-pinane (3), chlorolimonene (5), menthane (6), and n-hexane for the solubilization of β-carotenoids, vanillin, and rosmarinic acid. COSMO-RS integrates a quantum chemistry approach that allows the calculation of various properties as the relative solubility of a compound in several solvents. The analysis of the σ-profile and σ-potential of the components in the mixture (compounds and solvents) gives important information about the molecules that can be used to predict possible interactions in the fluid phase. Table 1 shows the COSMO-RS results.

Table 1.
Solubility prediction of β-carotenoids, vanillin, and rosmarinic acid.
Predictions indicate that chloropinane (2) solubilizes 60% fewer catenoids than cis-pinane (3), its non-halogenated analogue. However, chloromenthene (5) solubilizes the same proportion as menthene (6) (Table 1). For the solubilization of carotenoids, the predictions point to hexane as the best solvent followed by cis-pinane (3).
For vanillin, chloropinane (2) is predicted to solubilize two times more vanillin than its non-halogenated analogue while chloromenthene (5) solubilizes 3 times more vanillin than menthene (6) (Table 1).
Chloropinane (2) and chloromenthene (5) were predicted to outperform hexane 3 to 5 times, respectively, for vanillin solubilization (Table 1).
For rosmarinic acid, chloropinane (2) was predicted to be a better solvent than its non-halogenated analogue. This trend appears to be reversed with chloromenthene (5) since it was predicted to be a poorer solvent than menthene (6) (Table 1). Among the five solvents compared, menthene (6) would be the best solvent for the solubilization of rosmarinic acid (Table 1).
2.3. Solubility of β-Carotene, Vanillin, Rosmarinic Acid
The solubility of each compound in these different solvents at room temperature was realized by bead milling. Table 2 shows the solubility of carotenoids, vanillin, and rosmarinic acid in the five compared solvents. The results are expressed in solubility in g/L. The results are in agreement with the COSMO-RS prediction.

Table 2.
Solubility of β-caroten, vanillin, and rosmarinic acid.
The extraction of carotenoids depends on the solvent used, its polarity, and their solubility in the extraction solvents. Further, the solubility of carotenoids is related to physical and chemical properties, molecular structure and the nature of material particles, and operating conditions such as temperature, pressure, and density of solvents, which must be considered [10]. Considering the solubility of carotenoids, we evaluated the solubility of β-carotene with the five investigated solvents.
The highest carotenoids solubility was observed in the cis-pinane (3) as predicted by COSMO-RS (Table 1). Chloropinane (2) was six times less efficient than its non-halogenated analogue. However, it was six times more efficient than hexane for β-carotene solubilization (Table 2). Unlike chloropinane (2), chloromenthene (5) was 5 times better than its non-halogenated analogue and 15 times better than hexane (Table 2).
Vanillin was significantly better solubilized in chloropinane (2) than all other solvents including hexane (Table 2). Vanillin was over 9 times more soluble in chloropinane (2) than in cis-pinane (3) and 20 times more soluble in chloropinane (2) than in hexane (Table 2).
Chloromenthene (5) was the least efficient of all solvents including hexane for the solubilization of vanillin. Compared to menthene (6), chloromenthene (5) was 14 times less efficient for the solubilization of vanillin (Table 2).
For the solubilization of rosmarinic acid, chloropinane (2) and menthene (6) were the best solvents. These two solvents were 3.5 and 3.8 more efficient than hexane (Table 2). Chloropinane (2) was 10 times more efficient than cis-pinane, which confirms the affinity of certain molecules for halogenated solvents. On the other hand, chloromenthene (5) was half as good as its non-hydrogenated analogue (Table 2).
2.4. Energy Efficiency
Energy efficiency is a crucial point in a green chemical process (Green Chemistry Principle #6 [11]). The extraction must also follow this same principle to be considered green. Beyond the solubility of products in solvents, the energy required for the evaporation of these solvents must be part of the equation. The enthalpy of vaporization of 1 kg of the solvent (Evap) for the investigated solvent was calculated using specific heat, latent heat of vaporization, and the boiling point [12].
As shown in Table 3, chloropinane (2) and chloromenthene (5) required almost 30% less energy to be vaporized compared to n-hexane which is a good energy gain.

Table 3.
Energy efficiency.
3. Materials and Methods
3.1. Chemistry
All chemicals used were purchased from Aldrich (CA) (Manufactor, St. Louis, MI, USA) and used without further purification. FTIR spectra were recorded on a Cary 630 spectrometer (Agilent, Santa Clara, CA, USA). NMR spectra were recorded on Bruker® Avance III 400 MHz spectrometer (Bruker, Billerica, MA, USA) using TMS as an internal standard. High-resolution mass measurements were performed on Bruker® Daltonics’ micrOTOF (Bruker, Billerica, MA, USA) instrument in positive or negative electrospray. (1H-NMR, FTIR, 13C-NMR, and HRMS spectra: see Supplementary Materials).
3.1.1. Synthesis of Chloropinane (2)
To a solution of sodium borohydride (4.2 g, 0.11 mol) in THF (150 mL), β-pinene (30 g, 0.22 mol) was added at 0 °C. A solution of dimethyl sulfate (13.8 g, 0.11 mol) in THF (50 mL) was added dropwise to the reaction mixture over a period of 10 min at 0 °C and the reaction mixture was stirred for 12 h at r.t. Water (100 mL), 3 M NaOH (100 mL), and 30% H2O2 (100 mL) were added to the mixture cooled at 0 °C. The solution was stirred for 3 h followed by the addition of Et2O (150 mL). The aqueous phase was extracted with Et2O (3 × 50 mL). The combined organic phases were washed with water (3 × 100 mL) and brine (3 × 100 mL), dried over MgSO4, and concentrated under vacuum. To the obtained crud myrtenol dissolved in carbon tetrachloride (300 mL),triphenylphosphine (115 g, 0.44 mol) were added and the solution was refluxed for 24 h. Hexane (200 mL) was added to the reaction mixture, which was then cooled in an ice bath. The triphenylphosphine oxide which precipitated was filtered off under vacuum. The filtrate was then stored in the fridge for 24 h to precipitate the remaining triphenylphosphine oxide followed by a last filtration and solvent removal under vacuum. Fractional distillation afforded the desired chloropinane (2) (2-(chloromethyl)-6,6-dimethylbicyclo[3.1.1]heptane) as pale-yellow viscous liquid. Yield 14.8 g, (two step: 39%). B.p. 56–57 °C/1.5 mmHg. IR: v 2989, 2944, 2870, 1468, 1384, 1240, 730 cm−1. 1H-NMR (400 MHz, CDCl3, 25 °C); δ (ppm): 3.57–3.49 (m, 2H), 2.43–2.37 (m, 2H), 2.12–1.89 (m, 5H), 1.58–148 (m, 1H), 1.22 (s, 3H), 1.0 (s, 3H). 13C-NMR (101 MHz, CDCl3, 25 °C); δ (ppm): 50.15, 44.07, 44.0, 41.22, 38.53, 32.94, 27.81, 25.80, 23.20, 20.39. MS (m/z): 172, 174, 129, 123, 93, 81, 69, 55.
3.1.2. Synthesis of Chloromenthene (5)
To a stirred solution of (R)-(+)-limonene (5 g, 36.7 mmol) in CH2Cl2 (50 mL), Si02 (5 g) was added. The suspension was then stirred at room temperature for 15 min. A solution of SOCl2 (2.17 g, 18.4 mmol, 0.5 eq) in CH2Cl2 (5 mL) was added dropwise to the reaction mixture over a period of 10 min at 0 °C. The mixture was then stirred for 12 h at room temperature. The SiO2 was filtered and washed with CH2C12 (3 × 10 mL). To avoid any emulsion, the filtrate was evaporated and dissolved in EtOAC (100 mL) and then filtered. The EtOAc solution was washed with saturated aqueous solution of NaHCO3 (3 × 50 mL), water (3 × 50 mL), and brine (2 × 25 mL), then dried over MgS04 and concentrated under vacuum. Fractional distillation afforded the desired chloromenthene (5) (4-(2-chloropropan-2-yl)-1-methylcyclohexene) as pale-yellow viscous liquid. Yield: 3.3 g (52%), B.p. = 47–48 °C/1.5 mmHg. IR: v 2972, 2929, 2836, 1455, 1440, 1386, 1369, 1224, 1118, 801, 786 cm−1. 1H-NMR (400 MHz, CDCl3, 25 °C); δ (ppm): 5.39 (s, 1H), 2.11–2.69 (m, 1H), 2.06–2.01 (m, 3H), 1.99–1.90 (m, 1H), 1.79–178 (m, 1H), 1.67 (s, 3H), 1.61 (s, 3H), 1.56 (s, 3H), 1.46–1.38 (m, 1H). 13C-NMR (101 MHz, CDCl3, 25 °C); δ (ppm): 133.94, 120.12, 74.62, 46.47, 30.91, 30.71, 29.74, 27.44, 24.81, 23.24. MS (m/z) 172, 174, 136, 121, 107, 93, 67.
3.2. Prediction of Solubility
Solubility parameters of pinane, chloropinane, menthene, chlorolimonene, and n-hexane to dissolve beta-carotene, vanillin, and rosmarinic acid were investigated using COSMO-RS software. The chemical structures of the solvents and solutes were transformed by JChemPaint version 3.3 (GitHub Pages, San Francisco, CA, USA) to their simplified molecular input line entry syntax (SMILES) notations, which were subsequently used to calculate the solubility parameters of solvents and compounds.
The Conductor-like Screening Model for Real Solvents (COSMO-RS) uses a statistical thermodynamic approach based on the result of quantum chemical calculations for an understanding of the dissolving mechanism. COSMO-RS combines quantum chemical considerations and statistical thermodynamics to determine and predict the thermodynamic properties without experimental data. The COSMO-RS developed by Klamt is known as a powerful method for molecular description and solvent screening based on a quantum-chemical approach [6].
COSMO-RS is a two-step procedure including microscopic and macroscopic steps. In the first step, the COSMO model is applied to simulate a virtual conductor environment for the molecule of interest. The molecule is embedded into a virtual conductor. In such an environment, the molecule induced polarization charge density is on its surface. Thus, during the quantum calculation self-consistency algorithm the solute molecule is converged to its energetically optimal state in the conductor with respect to its electron density and geometry. The standard quantum chemical methods, triple zeta valence polarized basis set (TZVP), were used in this study.
The second step used the statistical thermodynamic calculation. This polarization charge density was used for the quantification of the interaction energy of the pair-wise interacting surface segments with regard the most important molecular interaction modes, i.e., electrostatics and hydrogen bonding. The 3D distribution of the polarization charges on the surface of each molecule was converted into a surface composition function (σ-profile). Such a σ-profile provided detailed information about the molecular polarity distribution. The thermodynamics of the molecular interactions that were based on the obtained σ-profile were then used to calculate the chemical potential of the surface segment (σ-potential) using the COSMOthermX program (version C30 release 13.01). The σ-potential described the likeliness of the compound being able to interact with the solvents with polarity and hydrogen bonds. The part of the negative charge of the molecule was located on the right side (acceptor hydrogen bonds) with positive σ values, while the part of the positive charged was located on the left side (donor hydrogen bonds) with negative σ values. Generally, the region σ ± 0.01 e/A2 was considered to be non-polar or weakly polar. The σ-profile and the σ-potential were used for interpreting the affinity of the solvent for surface polarity, to understand the interaction between the compound and solvent in mixed state and to estimate the thermodynamic properties of the system. In addition, the COSMOthermX also allows the determination of the relative solubility between the solid compound and the liquid solvent in terms of the logarithm of the solubility converted to solubility in g/L.
3.3. Test of Solubility
Solubilization by bead milling was performed using Precellys 24 (Bertin Technology, Ozyme, Montigny-le-Bretonneux, France) operating in a 2 mL tube with 1 g of ceramic beads. An amount of 100 mg of compounds was mixed with 1 mL of the investigated solvent and submitted in a drive tube operating at 6500 rpm during intervals of 2 × 20 s. After extraction, the beads were filtered before solid/liquid separation by centrifugation. The samples were stored at −20 °C until analysis. All experiments were carried out in triplicate.
The total content of each compound was measured spectrophotometrically (Biochrom Libra S22 UV/Vis Spectrophotometer, Cambridge, United Kingdom) in a 1 cm optical path-length quartz cell at the maximum wavelength of each compound in each extract using DMSO as a blank. The Beer–Lambert law was used to determine the carotenoid, vanillin, and rosmarinic acid concentration in each extract using a calibration curve prepared using β-carotene, vanillin, and rosmarinic acid standard. The straight calibration curve of absorbance versus compound concentration (mgL−1) was reliant on the Beer–Lambert law. Finally, the quantity of compound dissolve in each solvent was calculated and expressed as g/L.
4. Conclusions
In the present communication, chloropinane (2) and chloromenthene (5), which can be obtained from pinene and limonene, two products derived from plant biomass and more especially byproducts, were investigated as potential biobased-solvents for the extraction of β-carotenoids, vanillin, and rosmarinic acid.
Compared to their non-halogenated analogs and n-hexane, chloropinane (2) and chloromenthene (5) have shown a good ability to serve as non-fossil origin solvents. Prediction tests as well as experimental solubility tests of β-carotenoids, vanillin, and rosmarinic acid have shown that these halogenated products have the potential to replace n-hexane for the extraction of such natural products.
The synthesis method of the two chloro- derivatives could be optimized and improved with less carbon footprint to ideally be as green as possible. This first investigation of these solvents does not clearly prove their green and sustainable character. Safety studies regarding their bioaccumulation potential and their ecotoxicological profile will be necessary.
Supplementary Materials
The following are available online. FTIR, 1H-NMR, 13C-NMR, and HRMS spectra of compound 2 and 5.
Author Contributions
Conceptualization, M.T.; methodology, A.-S.F.-T., F.C., and M.T.; formal analysis, A.-S.F.-T., F.C., and M.T.; investigation, A.-S.F.-T., F.C., and M.T.; writing—original draft preparation, A.-S.F.-T., F.C., and M.T.; writing—review and editing, A.-S.F.-T., F.C., and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or supplementary material.
Acknowledgments
M.T. thanks J.A. Doiron for his help during the distillation of the two synthesized products and F.J. Ndongou Moutombi for her help with the MS analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tobiszewski, M.; Tsakovski, S.; Simeonov, V.; Namieśnik, J.; Pena-Pereira, F. A solvent selection guide based on chemometrics and multicriteria decision analysis. Green Chem. 2015, 17, 4773–4785. [Google Scholar] [CrossRef]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Varón, E.Y.; Selka, A.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Touaibia, M.; Chemat, F. Solvent from forestry biomass. Pinane a stable terpene derived from pine tree byproducts to substitute n-hexane for the extraction of bioactive compounds. Green Chem. 2016, 18, 6596–6608. [Google Scholar] [CrossRef]
- Selka, A.; Levesque, N.A.; Foucher, D.; Clarisse, O.; Chemat, F.; Touaibia, M. A Comparative Study of Solvent-Free and Highly Efficient Pinene Hydrogenation over Pd on Carbon, Alumina, and Silica Supports. Org. Process. Res. Dev. 2017, 21, 60–64. [Google Scholar] [CrossRef]
- Moutombi, F.J.N.; Selka, A.; Fabiano-Tixier, A.-S.; Foucher, D.; Clarisse, O.; Chemat, F.; Touaibia, M. Highly selective solvent-free hydrogenation of pinenes to added-value cis-pinane. Comptes Rendus Chim. 2018, 21, 1035–1042. [Google Scholar] [CrossRef]
- Klamt, A. Prediction of the mutual solubilities of hydrocarbons and water with COSMO-RS. Fluid Phase Equilibria 2003, 206, 223–235. [Google Scholar] [CrossRef]
- Moutombi, F.J.N.; Fabiano-Tixier, A.-S.; Clarisse, O.; Chemat, F.; Touaibia, M. Partial and Total Solvent-Free Limonene’s Hydrogenation: Metals, Supports, Pressure, and Water Effects. J. Chem. 2020, 2020, 5946345. [Google Scholar] [CrossRef]
- Beckmann, J.; Duthie, A.; Grassmann, M. Synthesis and structure of some cis-and trans-myrtanylstannanes. J. Organomet. Chem. 2009, 694, 161–166. [Google Scholar] [CrossRef]
- De Mattos, M.C.S.; Sanseverino, A.M. A Convenient and Simplified Preparation of Both Enantiomers of?-Terpinyl Chloride. Synth. Commun. 2000, 30, 1975–1983. [Google Scholar] [CrossRef]
- Arvayo-Enríquez, H.; Mondaca-Fernández, I.; Gortárez-Moroyoqui, P.; López-Cervantes, J.; Rodríguez-Ramírez, R. Carotenoids extraction and quantification: A review. Anal. Methods 2013, 5, 2916–2924. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2009, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Sicaire, A.-G.; Vian, M.; Fine, F.; Joffre, F.; Carré, P.; Tostain, S.; Chemat, F. Alternative Bio-Based Solvents for Extraction of Fat and Oils: Solubility Prediction, Global Yield, Extraction Kinetics, Chemical Composition and Cost of Manufacturing. Int. J. Mol. Sci. 2015, 16, 8430–8453. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).