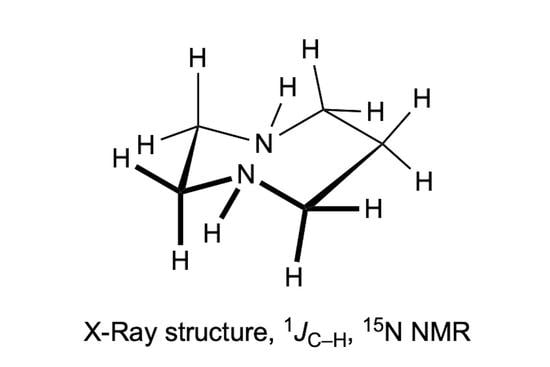
Homopiperazine (Hexahydro-1,4-diazepine)
Abstract
1. Introduction
2. Results
3. Experimental
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bleier, L. Bildung secundärer Basen aus Aethylendiamin. Ber. Dtsch. Chem. Ges. 1899, 32, 1825–1830. [Google Scholar] [CrossRef]
- Howard, C.C.; Marckwald, W. Ueber das Bistrimethylendiimin. Ber. Dtsch. Chem. Ges. 1899, 32, 2038–2042. [Google Scholar] [CrossRef]
- Poppelsdorf, F.; Myerly, R.C. A novel synthesis of homopiperazine and its monomethyl derivatives. J. Org. Chem. 1961, 26, 131–134. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, J.; Nam, S.; Jeong, S.; Yoon, Y. NMR study of carbon dioxide absorption in aqueous potassium carbonate and homopiperazine blend. Energy Fuels 2012, 26, 1449–1458. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Y.; Wang, L.; Zhang, F.; Fan, Z.; Cao, T.; Cao, Y.; Zhu, H.; He, X.; Deng, B.; et al. Effect of carbon-skeleton isomerism on the dielectric properties and proton conduction of organic cocrystal compounds assembled from 1,2,4,5-benzenetetracarboxylic acid and piperazine derivatives. New J. Chem. 2019, 43, 11099–11112. [Google Scholar] [CrossRef]
- Du, Y.; Li, H.; Wang, Z.; Zhang, M.; Liu, K.; Liu, Y.; Chen, R.; Wang, L. Supramolecular assemblies of bi-component molecular solids formed between homopiperazine and organic acids. J. Mol. Struct. 2019, 1196, 828–835. [Google Scholar] [CrossRef]
- Guo, W.; Fu, X.; Chen, J. Supramolecular adducts of mesocyclic diamines with various carboxylic acids: Charge-assisted hydrogen-bonding in molecular recognition. J. Saudi Chem. Soc. 2020, 24, 885–895. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Shi, C.; Zhang, W. Distinct room-temperature dielectric transition in a perchlorate-based organic-inorganic hybrid perovskite. Dalton Trans. 2017, 46, 16774–16778. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Huang, R.-K.; Chen, S.-L.; He, C.-T.; Yu, Z.-H.; Ye, Y.-M.; Zhang, W.-X.; Chen, X.-M. Metal-free molecular perovskite high-energetic materials. Cryst. Growth Des. 2020, 20, 1891–1897. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Guo, F.; Li, J.; Zeng, H.; Lin, Z. Extra-large-pore metal sulfate-oxalates with diamondoid and zeolitic frameworks. Inorg. Chem. Commun. 2018, 93, 33–36. [Google Scholar] [CrossRef]
- Sahbani, T.; Sta, W.S.; Al-Deyab, S.S.; Rzaigui, M. Homopiperazine-1,4-diium bis[hexaaquacobalt(II)] trisulfate. Acta Crystallogr. Sect. E 2011, 67, m1079. [Google Scholar] [CrossRef]
- Natarajan, S. Hydro/solvothermal synthesis and structures of new zinc phosphates of varying dimensionality. Inorg. Chem. 2002, 41, 5530–5537. [Google Scholar] [CrossRef]
- Wilkinson, H.S.; Harrison, W.T.A. Homopiperazinium bis(dihydrogenarsenate). Acta Crystallogr. Sect. E 2006, 62, m1397–m1399. [Google Scholar] [CrossRef]
- Hemissi, H.; Rzaigui, M.; Al-Deyab, S.S. Bis(homopiperazine-1,4-diium) cyclotetraphosphate-telluric acid (1/2). Acta Crystallogr. Sect. E 2010, 66, o2712. [Google Scholar] [CrossRef] [PubMed]
- Ameur, I.; Abid, S.; Besbes-Hentati, S.; Al-Deyab, S.S.; Rzaigui, M. Structural, vibrational, thermal and electrochemical studies of a cyclohexaphosphate complex, (C5H14N2)2Cd2Cl2P6O18•4H2O. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 1703–1712. [Google Scholar] [CrossRef]
- Dhieb, A.C.; Dridi, I.; Mathlouthi, M.; Rzaigui, M.; Smirani, W. Structural physico chemical studies and biological analyses of a cadmium cluster complex. J. Cluster Sci. 2018, 29, 1123–1131. [Google Scholar] [CrossRef]
- Said, M.; Boughzala, H. Structural characterization and physicochemical features of new coordination polymer homopiperazine-1,4-diium tetrachlorocadmate(II). J. Mol. Struct. 2020, 1220, 128696. [Google Scholar] [CrossRef]
- Mao, L.; Guo, P.; Kepenekian, M.; Hadar, I.; Katan, C.; Even, J.; Schaller, R.D.; Stoumpos, C.C.; Kanatzidis, M.G. Structural diversity in white-light-emitting hybrid lead bromide perovskites. J. Am. Chem. Soc. 2018, 140, 13078–13088. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Mironov, A.V.; Bykov, M.A.; Grigorieva, A.V.; Wei, Z.; Dikarev, E.V.; Shevelkov, A.V. Assembling polyiodides and iodobismuthates using a template effect of a cyclic diammonium cation and formation of a low-gap hybrid iodobismuthate with high thermal stability. Molecules 2020, 25, 2765. [Google Scholar] [CrossRef]
- Almond, P.M.; Deakin, L.; Mar, A.; Albrecht-Schmitt, T.E. Hydrothermal syntheses, structures, and magnetic properties of the U(IV) fluorides (C5H14N2)2U2F12•5H2O and (NH4)7U6F31. J. Solid State Chem. 2001, 158, 87–93. [Google Scholar] [CrossRef]
- Doran, M.B.; Norquist, A.J.; O’Hare, D. Exploration of composition space in templated uranium sulfates. Inorg. Chem. 2003, 42, 6989–6995. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-M.; Du, M.; Bu, X.-H. Bis(1,4-diazacycloheptane-N,N’)nickel(II) perchlorate. Acta Crystallogr. Sect. E 2001, 57, m280–m282. [Google Scholar] [CrossRef]
- Klai, K.; Kaabi, K.; Jelsch, C.; Wenger, E.; Lefebvre, F.; Ben Nasr, C. A Hirshfeld surface analysis, synthesis, structure and characterization of a new Ni(II) diamagnetic complex with the bidentate ligand homopiperazine. J. Mol. Struct. 2017, 1148, 412–420. [Google Scholar] [CrossRef]
- Xing, Z.; Yin, H.-B. Crystal structure of (1,4-diazepane)4CuII2(μ-Cl)10CuI6, C20H48Cl10Cu8N8. Z. Kristallogr. NCS 2019, 243, 391–392. [Google Scholar] [CrossRef]
- Ling, E.C.H.; Allen, G.W.; Hambley, T.W. DNA binding of a platinum(II) complex designed to bind interstrand but not intrastrand. J. Am. Chem. Soc. 1994, 116, 2673–2674. [Google Scholar] [CrossRef]
- Ali, M.S.; Powers, C.A.; Whitmire, K.H.; Guzman-Jimenez, I.; Khokhar, A.R. Synthesis, characterization, and representative crystal structure of lipophilic platinum(II) (homopiperazine)carboxylate complexes. J. Coord. Chem. 2001, 52, 273–287. [Google Scholar] [CrossRef]
- Ali, M.S.; Mukhopadhyay, U.; Shirvani, S.M.; Thurston, J.; Whitmire, K.H.; Khokhar, A.R. Homopiperazine platinum(II) complexes containing substituted disulfide groups: Crystal structure of [PtII(homopiperazine)(diphenylsulfide)Cl]NO3. Polyhedron 2002, 21, 125–131. [Google Scholar] [CrossRef]
- Ali, M.S.; Longoria, E., Jr.; Ely, T.O.; Whitmire, K.H.; Khokhar, A.R. Homopiperazine Pt(II) adducts with DNA bases and nucleosides: Crystal structure of [PtII(homopiperazine)(9-ethylguanine)2](NO3)2. Polyhedron 2006, 25, 2065–2071. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, S1–S19. [Google Scholar] [CrossRef]
- Fonari, M.S.; Antal, S.; Castañeda, R.; Ordonez, C.; Timofeeva, T.V. Crystalline products of CO2 capture by piperazine aqueous solutions. CrystEngComm 2016, 18, 6282–6289. [Google Scholar] [CrossRef]
- Sui, Y.; Hu, R.-H.; Luo, Z.-G.; Wen, J.-W.; Zhong, L.-J. Crystal structure and switchable dielectric properties of singly protonated homopiperazinium perchlorate. Asian J. Chem. 2015, 27, 2876–2878. [Google Scholar] [CrossRef]
- Greiser, J.; Kühnel, C.; Görls, H.; Weigand, W.; Freesmeyer, M. N,1,4-Tri(4-alkoxy-2-hydroxybenzyl)-DAZA: Efficient one-pot synthesis and labelling with 68Ga for PET liver imaging in ovo. Dalton Trans. 2018, 47, 9000–9007. [Google Scholar] [CrossRef]
- Coyle, J.; Downard, A.J.; Nelson, J.; McKee, V.; Harding, C.J.; Herbst-Irmer, R. Electrochemistry of a labile average-valence dicopper system. Dalton Trans. 2004, 15, 2357–2363. [Google Scholar] [CrossRef]
- Mohamed, Y.A.H.; Chang, C.-J.; McLaughlin, J.L. Cactus alkaloids. XXXIX. A glucotetrahydroisoquinoline from the Mexican cactus Pterocereus gaumeri. J. Nat. Prod. 1979, 42, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Albrand, J.P.; Cogne, A.; Gagnaire, D.; Robert, J.B. Analyse des spectres de RMN d’imidazolidines. Utilisation des satellites 13C. Tetrahedron 1971, 27, 2453–2461. [Google Scholar] [CrossRef]
- Chertkov, V.A.; Sergeyev, N.M. 13C–1H coupling constants in cyclohexane. J. Am. Chem. Soc. 1977, 99, 6750–6752. [Google Scholar] [CrossRef]
- Dokalik, A.; Kalchhauser, H.; Mikenda, W.; Schweng, G. NMR spectra of nitrogen-containing compounds. Correlations between experimental and GIAO calculated data. Magn. Reson. Chem. 1999, 37, 895–902. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
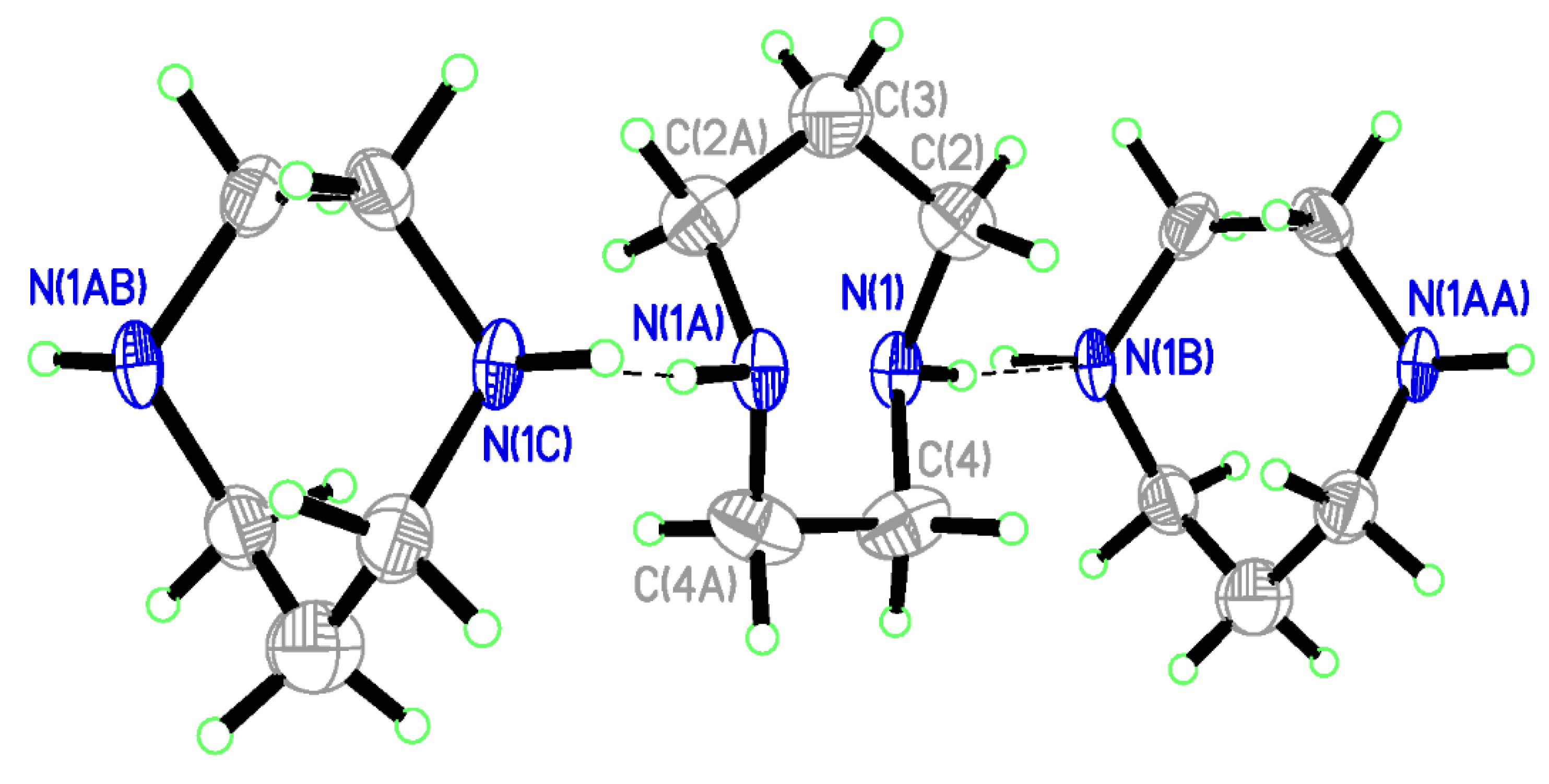

| D—H…A | D—H | H…A | D…A | D—H…A |
|---|---|---|---|---|
| N(1)–H(1)…N(1B) | 0.977(7) | 2.21(3) | 3.189(4) | 175(4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Sonecha, D.K.; Slawin, A.M.Z. Homopiperazine (Hexahydro-1,4-diazepine). Molbank 2021, 2021, M1200. https://doi.org/10.3390/M1200
Aitken RA, Sonecha DK, Slawin AMZ. Homopiperazine (Hexahydro-1,4-diazepine). Molbank. 2021; 2021(2):M1200. https://doi.org/10.3390/M1200
Chicago/Turabian StyleAitken, R. Alan, Dheirya K. Sonecha, and Alexandra M. Z. Slawin. 2021. "Homopiperazine (Hexahydro-1,4-diazepine)" Molbank 2021, no. 2: M1200. https://doi.org/10.3390/M1200
APA StyleAitken, R. A., Sonecha, D. K., & Slawin, A. M. Z. (2021). Homopiperazine (Hexahydro-1,4-diazepine). Molbank, 2021(2), M1200. https://doi.org/10.3390/M1200