Abstract
Using environmentally friendly conditions, the nucleophilic ring-opening reaction of 6-azabicyclo[3.1.0]hex-3-en-2-ol with 1-methyl-1H-tetrazole-5-thiol provided a novel thiol-incorporated aminocyclopentitol, (1R,4S,5S)-5-((3-hydroxypropyl)amino)-4-((1-methyl-1H-tetrazol-5-yl)thio)cyclopent-2-en-1-ol, in excellent yield (95%). The newly synthesized compound was analyzed and characterized via 1H, 13C-NMR, HSQC, and mass spectral data.
1. Introduction
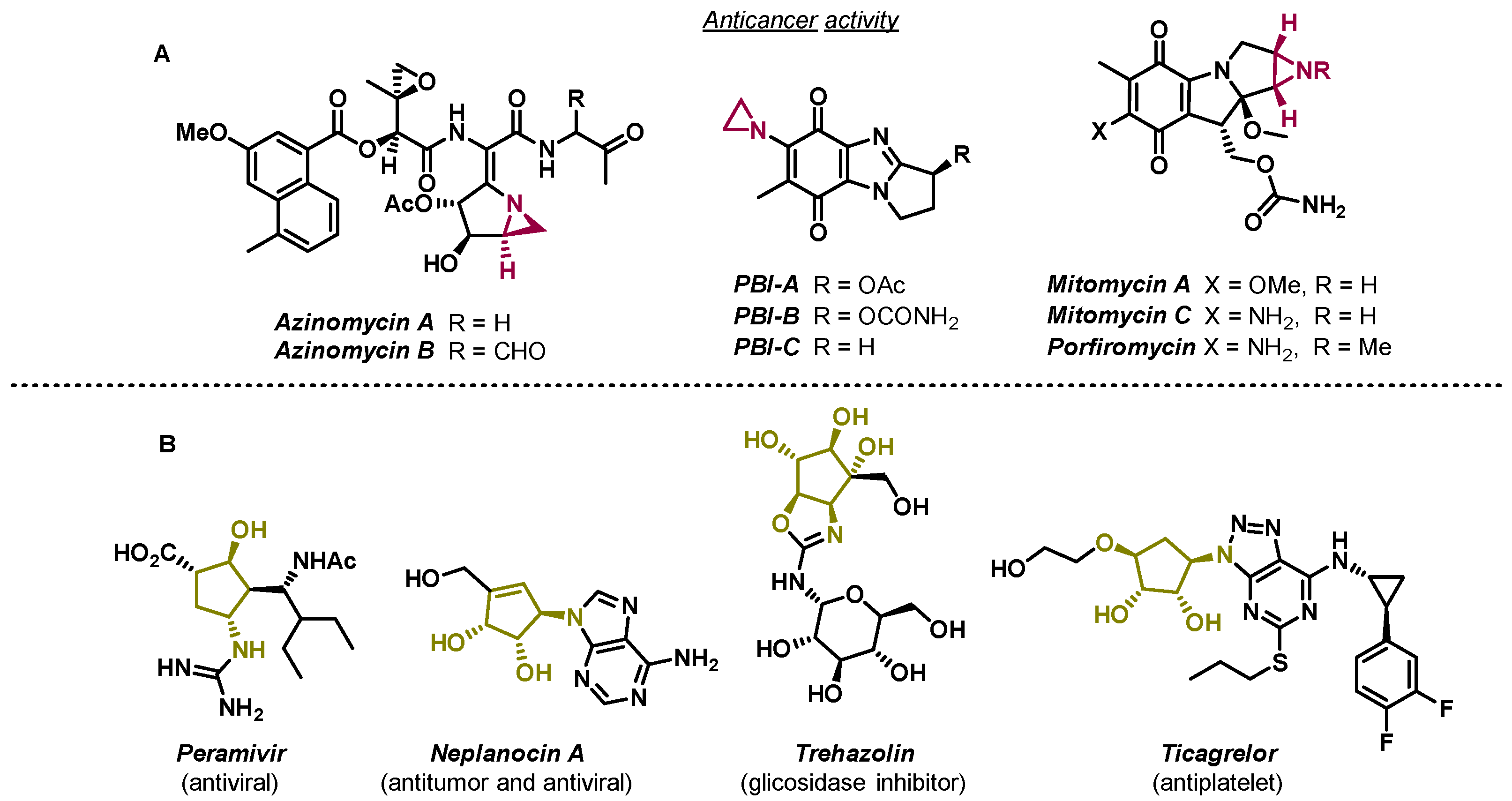
Aziridines are recurrent motifs in anticancer compounds (Figure 1A) and are useful building blocks in organic synthesis, largely due to their ring strain [1].
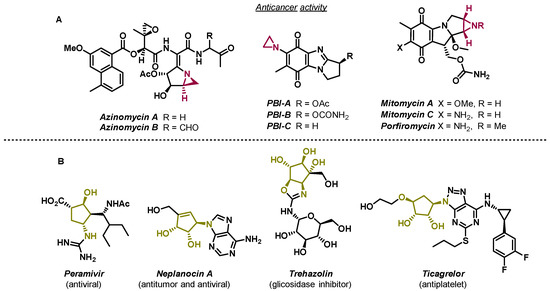
Figure 1.
Examples of chemical structures of bioactive compounds containing aziridines (A) and important biological compounds containing the aminocyclopentitol scaffold (B) [2,3,4].
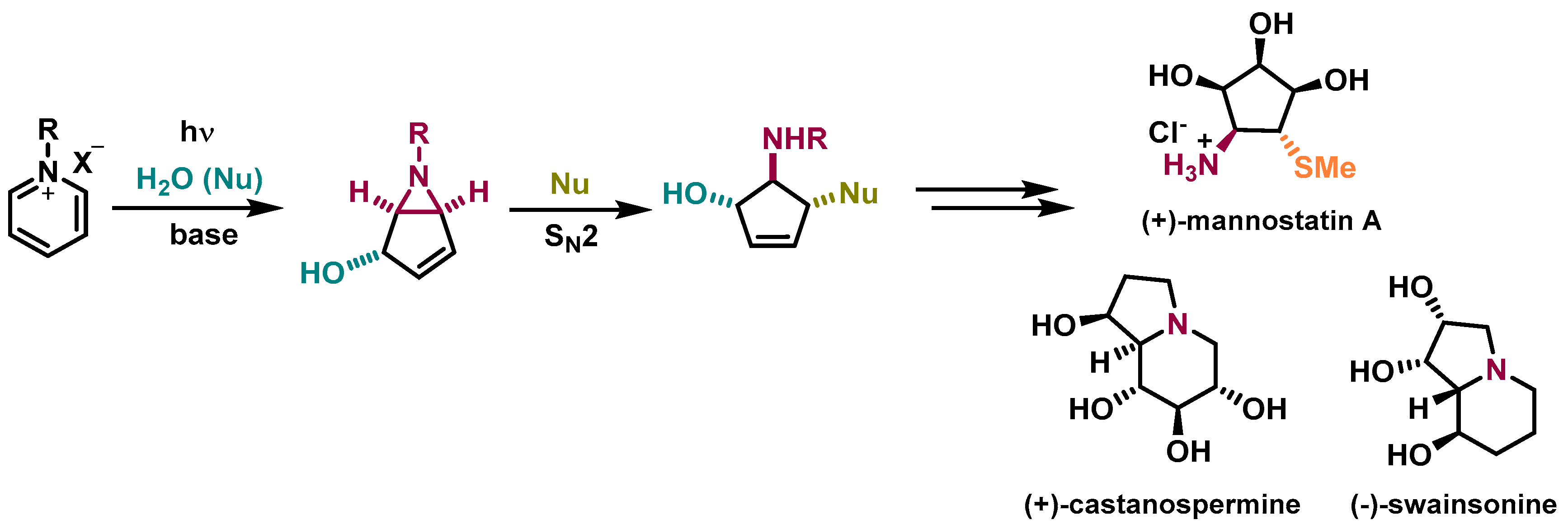
In 1972, Kaplan and co-workers implemented an innovative methodology to prepare 6-azabicyclo[3.1.0]hex-3-en-2-ol (bicyclic vinyl aziridines) via the photochemical conversion of pyridinium salts (Scheme 1) [5]. Bicyclic vinyl aziridines are useful intermediates to access aminocyclopentitols, a family of natural compounds known for being glycosidase inhibitors (Figure 1B) [6,7]. The synthetic methodology to prepare aminocyclopentitols involves the synthesis of bicyclic vinyl aziridines, followed by a ring-opening reaction to originate an aminocyclopentene, which after further functionalization originate the desired aminocyclopentitol. This methodology was developed by Mariano [8] and applied to the synthesis of several aminocyclopentitols, such as (+)-mannostatin A [9], (+)-castanospermine [10], and (-)-swainsonine [11] (Scheme 1).
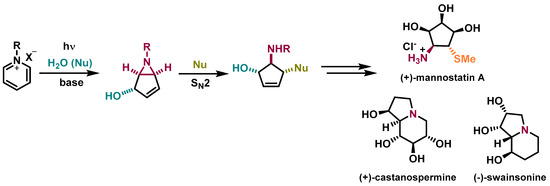
Scheme 1.
Synthetic methodology to prepare aminocyclopentitols by taking advantage of bicyclic vinyl aziridines prepared via photoreactions of pyridinium salts [9,10,11].
Mannostatin A is a natural product, first isolated from a soil microorganism Streptoverticillus, and is among the most potent inhibitors of class II α-mannosidase. The chemical structure of Mannostatin A contains a thiol functionality, responsible for the high affinity to the enzyme’s binding site [12]. Inhibitors of glycosidases are leading the drug discovery across cancer, and viral and bacterial infections. Mannostatin A and its analogs [13] have been used to study the inhibition of glycosidases to orientate the development of drug candidates [6,7].
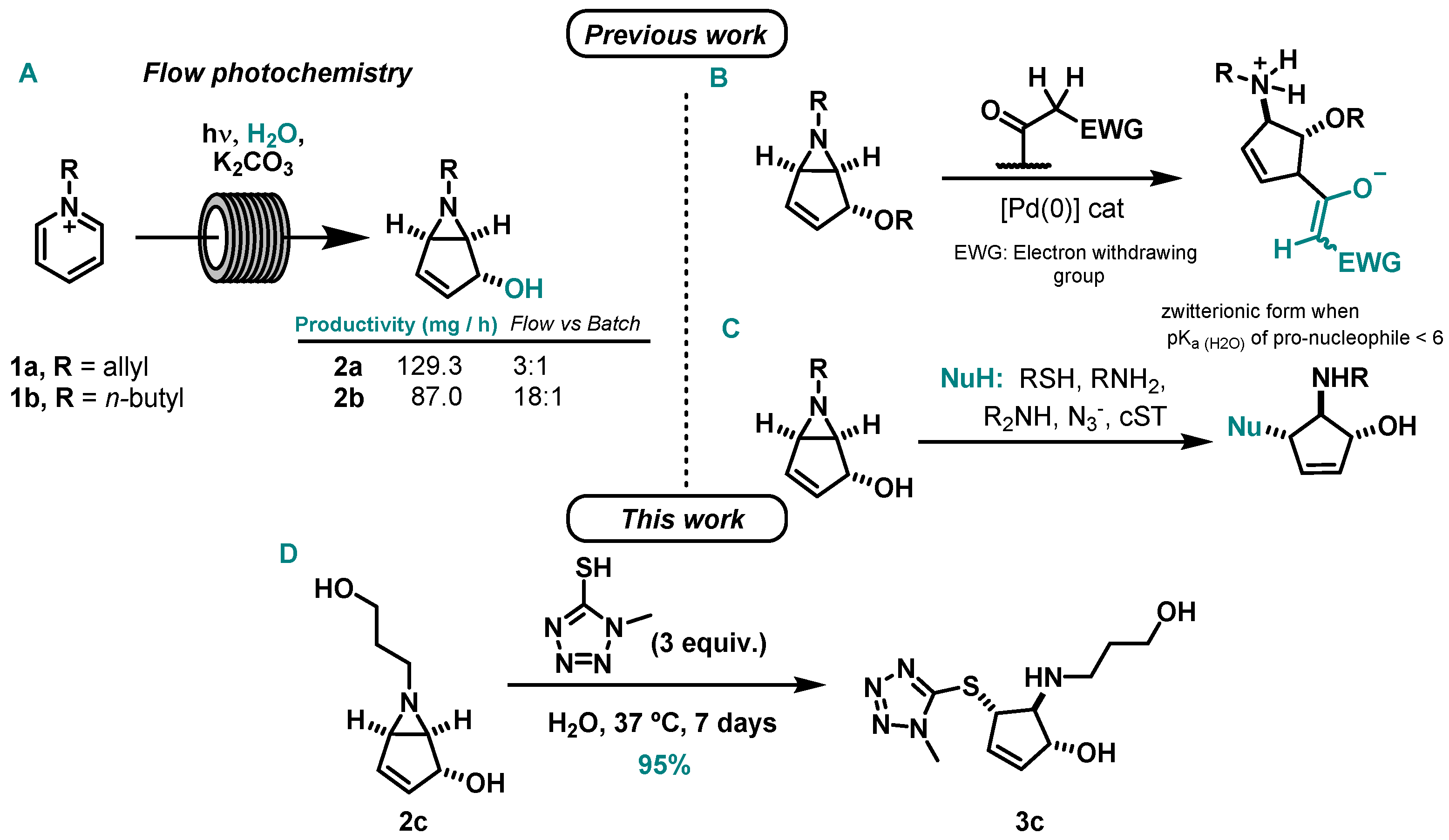
Our group studied the photochemical reactions of pyridinium salts to bicyclic vinyl aziridines under continuous-flow [14,15]. The implementation of flow enabled the synthesis of bicyclic vinyl aziridines with larger productivity when compared to reported batch methods [16] and also the achievement of a gram scale production [15] (Scheme 2A).
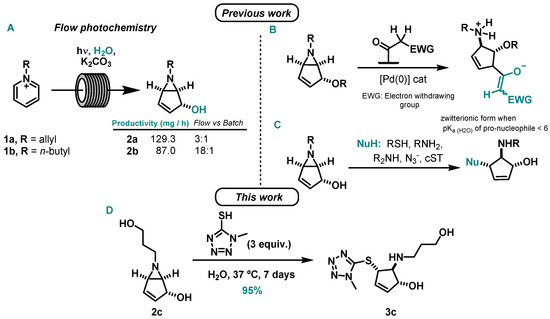
Scheme 2.
(A) Flow photocyclization of pyridinium salts [14,15], improvements over batch methodology [16,17]; previous work on (B) palladium-catalyzed allylic substitutions on bicyclic vinyl aziridines using carbon-based nucleophiles [18] and (C) nucleophilic ring-opening reactions of bicyclic vinyl aziridines with thiol and nitrogen nucleophiles [17]; (D) this work: nucleophilic ring-opening reaction of bicyclic vinyl aziridine ring (2c) by 1-methyl-1H-tetrazole-5-thiol to produce 3c.
We also studied several bicyclic vinyl aziridines transformations. In collaboration with G. Poli, we reported a palladium-catalyzed allylic substitutions using C-nucleophiles [18] (Scheme 2B). Additionally, we accomplished several ring-opening reactions using sulfur and nitrogen-based nucleophiles in an aqueous medium, including a bioconjugation with the peptide hormone salmon calcitonin (sCT) [17] (Scheme 2C). Within the reactions performed, the best yields were achieved for thiol-nucleophiles. In line with this work, we herein present the ring-opening reaction of 6-(3-hydroxypropyl)-6-azabicyclo[3.1.0]hex-3-en-2-ol by a thiol-based nucleophile, 1-methyl-1H-tetrazole-5-thiol (Scheme 2D).
2. Results and Discussion
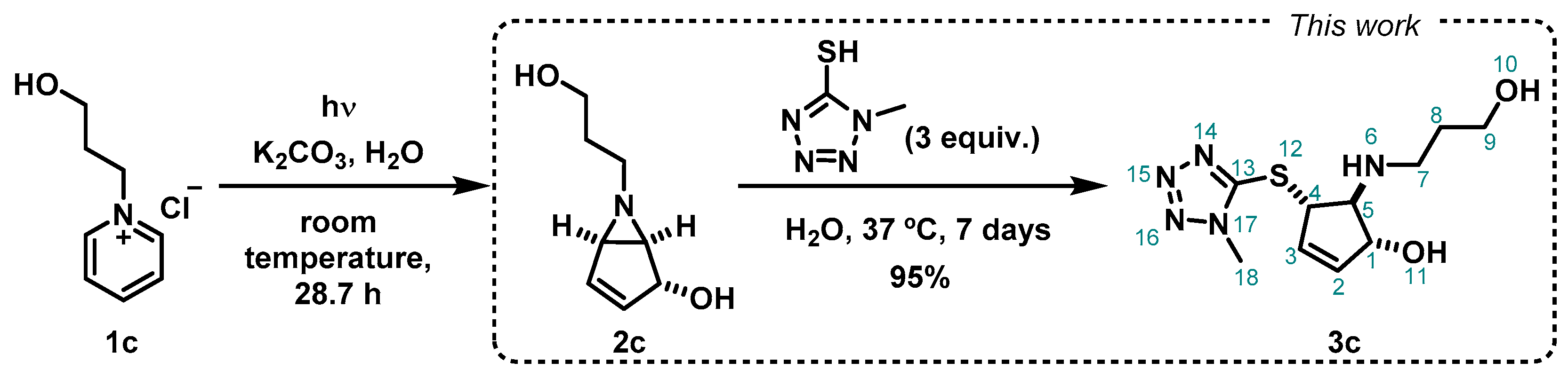
The starting material, 1-(3-hydroxypropyl)pyridin-1-ium chloride (1c), was prepared from pyridine and 3-chloropronanol following our reported method. 6-(3-hydroxypropyl)-6-azabicyclo[3.1.0]hex-3-en-2-ol (2c) was obtained by photohydration of the pyridinium salt 1c (Scheme 3), also taking advantage of our previous reported synthetic methodology [18]. In this work, the bicyclic vinyl aziridine 2c was subjected to thiol-nucleophilic attack by 1-methyl-1H-tetrazole-5-thiol, applying our reported optimized conditions [17]. An excess of the nucleophile (3 equivalents) was used under mild reaction conditions (37 °C, in water), and we obtained the product 3c as a brown oil, in excellent yield (95%) after purification by silica gel chromatography (Scheme 3). The ring-opening reaction occurs via the SN2 pathway in a regio- and stereospecific manner, and the nucleophile attacks in the less sterically hindered carbon of the aziridine moiety, as previously reported by Mariano [19] and Burger [20], and more recently also by us [17].
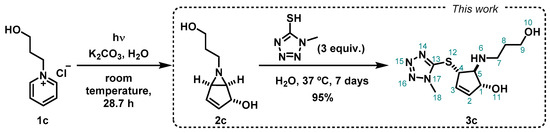
Scheme 3.
Synthetic pathway from photocyclization of 1-(3-hydroxypropyl)pyridin-1-ium chloride (1c) [18], followed by a nucleophilic attack to the bicyclic vinyl aziridine ring (2c) by 1-methyl-1H-tetrazole-5-thiol to produce 3c.
The product 3c was characterized by 1H-NMR, 13C-NMR, HSQC, and HRMS. By analyzing the 1H-NMR spectrum (Figure S1), we can observe characteristic peaks from product 3c: a singlet at 3.96 ppm, corresponding to the methyl group linked to the tetrazole ring (H-18), and multiplets corresponding to the geminal protons of the thioether at 4.30–4.29 ppm (H-4), the alcohol at 4.59–4.58 ppm (H-1), and the amine at 3.29–3.26 ppm (H-5). The signals for the hydroxypropyl chain can be observed as a quartet (J = 6.9 Hz, 2.77 ppm), a multiplet (1.76–1.67 ppm), and a triplet (J = 6.4 Hz, 3.60 ppm), corresponding to the protons vicinal to the amine (H-7), to the middle-chain methylene (H-8), and to the protons geminal to the hydroxyl group (H-9), respectively. Additionally, the 13C-NMR (Figure S2) shows the characteristic peaks from the tetrazole ring: a quaternary carbon at 153.32 ppm (C-13), which does not correlate with a proton signal in the HSQC (Figure S3), and the carbon from the methyl group at 33.87 ppm (C-18).
The product 3c can be further functionalized, since it has a primary and a secondary hydroxyl group. Moreover, 3c has a tetrazole ring which could lead to potential biological activity, since the tetrazole moiety can be found in different approved [21] and candidate drugs [22,23]. Accomplishing the synthesis of 3c contributed to expanding our previous aminocyclopentitols library [17].
3. Materials and Methods
All chemicals, reagents, and solvents were of analytical grade, purchased from commercial sources, namely, Merck (Algés, Portugal) and Alfa Aesar (Kandel, Germany) and were used without further purification. NMR spectra were obtained on a Bruker Fourier 300 spectrometer (Bruker BioSpin AG, Fallanden, Switzerland) using TopSpin(®) software (Bruker BioSpin GmbH, Rheinstetten, Germany). NMR experiments were performed in D2O at room temperature. Chemical shifts are given in parts per million (ppm); the terms m, s, d, t, and q represent multiplet, singlet, doublet, triplet, and quartet, respectively; and the coupling constants (J) are given in Hertz (Hz). High-resolution mass spectroscopy (HRMS) was performed in a LTQ Orbitrap XL mass spectrometer, Thermo Fischer Scientific, Bremen, Germany.
1-(3-Hydroxypropyl)pyridin-1-ium chloride (1c) and (1R,2R,5R)-6-(3-hydroxypropyl)-6-azabicyclo[3.1.0]hex-3-en-2-ol (2c) were prepared as previously described by us [18].
(1R,4S,5S)-5-((3-hydroxypropyl)amino)-4-((1-methyl-1H-tetrazol-5-yl)thio)cyclopent-2-en-1-ol 3c: To a solution of (1R,2R,5R)-6-(3-hydroxypropyl)-6-azabicyclo[3.1.0]hex-3-en-2-ol 2c (29.6 mg; 0.19 mmol) in distilled water (1 mL), 1-methyl-1H-tetrazole-5-thiol (60.1 mg; 0.57 mmol, 3 equiv.) was added. The reaction mixture was stirred at 37 ℃ and followed by TLC (eluent: dichloromethane/methanol, 9:1) until the complete disappearance of the starting material, observed after 7 days. The crude reaction was concentrated under reduced pressure and purified by silica gel chromatography eluting with dichloromethane, methanol, and triethylamine (9:1:0.1) to afford the ring-opening product 3c as a brown oil in 95% yield (49.23 mg).
1H-NMR (300 MHz, D2O) δ 5.95–5.90 (m, 2H, H-2 and H-3), 4.59–4.58 (m, 1H, H-1), 4.30–4.29 (m, 1H, H-4), 3.96 (s, 3H, H-18), 3.60 (t, J = 6.4 Hz, 2H, H-9), 3.29–3.26 (m, 1H, H-5), 2.77 (q, J = 6.9 Hz, 2H, H-7), 1.76-1.67 (m, 2H, H-8).
13C-NMR (100 MHz, D2O) δ 153.32 (C-13), 135.02 (C-3), 131.91 (C-2), 80.01 (C-1), 72.44 (C-5), 59.60 (C-9), 55.55 (C-4), 44.05 (C-7), 33.87 (C-18), 30.67 (C-8).
HRMS m/z calc. for C10H17N5O2S [M + H]+ 272.11757, obtained 272.11740.
4. Conclusions
We obtained (1R,4S,5S)-5-((3-hydroxypropyl)amino)-4-((1-methyl-1H-tetrazol-5-yl)thio)cyclopent-2-en-1-ol (3c) through bicyclic vinyl aziridine ring-opening reaction, with 1-methyl-1H-tetrazole-5-thiol. The reaction was executed under mild (37 ℃) and sustainable (water as reaction medium) conditions. The compound 3c was characterized using 1H NMR, 13C NMR, HSQC, and HRMS.
Supplementary Materials
The following are available online, Figure S1: 1H NMR spectrum; Figure S2: 13C-NMR spectrum; Figure S3: HSQC spectrum; Figure S4: HRMS.
Author Contributions
Conceptualization and supervision, F.S. and C.A.M.A.; investigation, M.A.G.F.; data curation and formal analysis, M.A.G.F. and F.S.; writing, M.A.G.F., F.S. and C.A.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação para a Ciência e a Tecnologia (FCT) (reference number: PTDC/QUI-QOR/32008/2017). The project leading to this application received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 951996.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chai, Z. Catalytic Asymmetric Transformations of Racemic Aziridines. Synthesis 2020, 52, 1738–1750. [Google Scholar] [CrossRef]
- Sweeney, J.B. Aziridines: Epoxides’ ugly cousins? Chem. Soc. Rev. 2002, 31, 247–258. [Google Scholar] [CrossRef]
- Košak, U.; Hrast, M.; Knez, D.; Maraš, N.; Črnugelj, M.; Gobec, S. Convenient syntheses of orthogonally protected aminocyclopentitols from aldopentoses. Tetrahedron Lett. 2015, 56, 529–531. [Google Scholar] [CrossRef]
- Marin, L.; Force, G.; Gandon, V.; Schulz, E.; Lebœuf, D. Aza-Piancatelli Cyclization as a Platform for the Preparation of Scaffolds of Natural Compounds: Application to the Total Synthesis of Bruceolline D. Eur. J. Org. Chem. 2020, 2020, 5323–5328. [Google Scholar] [CrossRef]
- Kaplan, L.; Pavlik, J.W.; Wilzbach, K.E. Photohydrataion of Pyridinium Ions. J. Am. Chem. Soc. 1972, 94, 3283–3284. [Google Scholar] [CrossRef]
- Delgado, A. Recent Advances in the Chemistry of Aminocyclitols. Eur. J. Org. Chem. 2008, 2008, 3893–3906. [Google Scholar] [CrossRef]
- Diaz, L.; Delgado, A. Medicinal Chemistry of Aminocyclitols. Curr. Med. Chem. 2010, 17, 2393–2418. [Google Scholar] [CrossRef]
- Yoon, U.C.; Quillen, S.L.; Mariano, P.S.; Swanson, R.; Stavinoha, J.L.; Bay, E. Electron transfer initiated photocyclizations of N-allylpridinium and quinolinium salts. Tetrahedron Lett. 1982, 23, 919–922. [Google Scholar] [CrossRef]
- Ling, R.; Mariano, P.S. A Demonstration of the Synthetic Potential of Pyridinium Salt Photochemistry by Its Application to a Stereocontrolled Synthesis of (+)-Mannostatin A 1. J. Org. Chem. 1998, 63, 6072–6076. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Song, L.; Mariano, P.S. A concise sequential photochemical-metathesis approach for the synthesis of (+)-castanospermine and possible uniflorine-A stereoisomers. Tetrahedron 2005, 61, 8888–8894. [Google Scholar] [CrossRef]
- Song, L.; Duesler, E.N.; Mariano, P.S. Stereoselective synthesis of polyhydroxylated indolizidines based on pyridinium salt photochemistry and ring rearrangement metathesis. J. Org. Chem. 2004, 69, 7284–7293. [Google Scholar] [CrossRef] [PubMed]
- Kawatkar, S.P.; Kuntz, D.A.; Woods, R.J.; Rose, D.R.; Boons, G.J. Structural basis of the inhibition of Golgi α-mannosidase II by mannostatin A and the role of the thiomethyl moiety in ligand-protein interactions. J. Am. Chem. Soc. 2006, 128, 8310–8319. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Ling, R.; Kim, A.; Mariano, P.S. A Versatile Approach to the Synthesis of (+) -Mannostatin A Analogues. J. Org. Chem. 2000, 65, 1574–1577. [Google Scholar] [CrossRef] [PubMed]
- Siopa, F.; António, J.P.M.; Afonso, C.A.M. Flow-Assisted Synthesis of Bicyclic Aziridines via Photochemical Transformation of Pyridinium Salts. Org. Process Res. Dev. 2018, 22, 551–556. [Google Scholar] [CrossRef]
- Fortunato, M.A.G.; Ly, C.-P.; Siopa, F.; Afonso, C.A.M. Process Intesification for the Synthesis of 6-Allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol from 1-Allylpyridinium Salt Using a Continuous UV-Light Photoflow Approach. Methods Protoc. 2019, 2, 67. [Google Scholar] [CrossRef]
- Colombo, C.; Pinto, B.M.; Bernardi, A.; Bennet, A.J. Synthesis and evaluation of influenza A viral neuraminidase candidate inhibitors based on a bicyclo[3.1.0]hexane scaffold. Org. Biomol. Chem. 2016, 14, 6539–6553. [Google Scholar] [CrossRef]
- Vale, J.R.; Siopa, F.; Branco, P.S.; Afonso, C.A.M. Ring Opening of 6-Azabicyclo-[3.1.0]hex-3-en-2-ols in Water under Mild Conditions. Eur. J. Org. Chem. 2016, 2016, 2048–2053. [Google Scholar] [CrossRef]
- Oliveira, J.A.C.; Kiala, G.; Siopa, F.; Bernard, A.; Gontard, G.; Oble, J.; Afonso, C.A.M.; Poli, G. Palladium-catalyzed allylic substitution between C-based nucleophiles and 6-azabicyclo[3.1.0]-hex-3-en-2-oxy derivatives: A new selectivity paradigm. Tetrahedron 2020, 76, 131182. [Google Scholar] [CrossRef]
- Ling, R.; Yoshida, M.; Mariano, P.S. Exploratory investigations probing a preparatively versatile, pyridinium salt photoelectrocyclization-solvolytic aziridine ring opening sequence. J. Org. Chem. 1996, 61, 4439–4449. [Google Scholar] [CrossRef]
- Acar, E.A.; Glarner, F.; Burger, U. Aminocyclopentitols from N-Alkylpyridinium Salts: A photochemical approach. Helv. Chim. Acta 1998, 81, 1095–1104. [Google Scholar] [CrossRef]
- Gleiter, C.H.; Jägle, C.; Gresser, U.; Mörike, K. Candesartan. Cardiovasc. Drug Rev. 2004, 22, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, A.G.S.; Hull, C.M.; Parker, J.E.; Garvey, E.P.; Hoekstra, W.J.; Moore, W.R.; Schotzinger, R.J.; Kelly, D.E.; Kellya, S.L. The clinical candidate VT-1161 is a highly potent inhibitor of candida albicans CYP51 but fails to bind the human enzyme. Antimicrob. Agents Chemother. 2014, 58, 7121–7127. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Fothergill, A.W.; Iqbal, N.; Bolden, C.B.; Grossman, N.T.; Garvey, E.P.; Brand, S.R.; Hoekstra, W.J.; Schotzinger, R.J.; Ottinger, E.; et al. The Investigational Fungal Cyp51 Inhibitor VT-1129 Demonstrates Potent In Vitro Activity against Cryptococcus neoformans and Cryptococcus gattii. Antimicrob. Agents Chemother. 2016, 60, 2528–2531. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).