Abstract
Reaction of 4-oxo-3,4-dihydroquinazoline-2-carbaldehyde with hydroxylamine hydrochloride (1.1 equiv) in the presence of K2CO3 (1 equiv) gave (E)-4-oxo-3,4-dihydroquinazoline-2-carbaldehyde oxime (8) in 58% yield. The compound was fully characterized and the conformation of the oxime was supported by single crystal x-ray diffractometry.
1. Introduction
Quinazolines are important aromatic N-heterocycles that have wide pharmaceutical applications. Among the 6-membered aromatic nitrogen-containing heterocycles, quinazolines rank 3rd in the most frequently used U.S. FDA-approved drugs [1]. Examples of quinazoline-containing drugs are the anticancer drug erlotinib and the antihypertensive prazosin (Figure 1). The chemistry and applications of quinazolines have been reviewed [2].
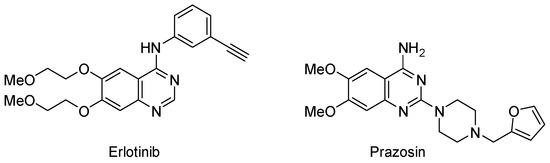
Figure 1.
Quinazolines in drugs.
2. Results and Discussion
Our interest in quinazolines began with 3′,5′-dichloro-1H-spiro(quinazoline-2,4′-[1,2,6]thiadiazin)-4(3H)-ones 1 that can be synthesized in 3 steps starting from tetrachlorothiadiazine 2 [3] (Scheme 1). Interestingly, spirocycle 1a (R = H) can also be decomposed to 4-oxo-3,4-dihydroquinazoline-2-carbonitrile (3) [4] (Scheme 1). More recently, while investigating the stability of 4-chlorobenzo[e][1,2,6]thiadiazino[3,4-b][1,4]diazepin-10(11H)-one (4), we found that upon heating in MeOH/AcOH (90:10), it was converted in 92% yield to the isomeric 2-(4-chloro-1,2,5-thiadiazol-3-yl)quinazolin-4(3H)-one (5) [5] (Scheme 1). We attributed this transformation to an acid-catalyzed nucleophilic addition to the thiadiazine 4 followed subsequent Wagner–Meerwein shifts leading to the observed ring contractions [5].
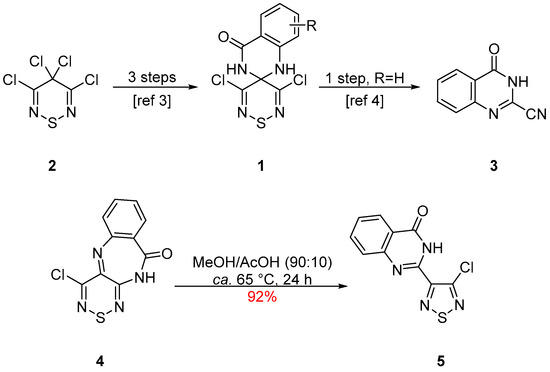
Scheme 1.
Synthesis of spirocycles 1 and quinazolinone 3 and isolation of thiadiazole 5 from diazepine 4.
We were interested in developing an independent synthesis for thiadiazole 5 to investigate its chemistry. The proposed independent synthesis started from 2-aminobenzamide 6, which can be converted to quinazolinone-2-carbaldehyde 7 in two steps with 51% overall yield [6] (Scheme 2). The conversion of aldehyde 7 into oxime 8, followed by the addition of cyanide should give hydroxylamine 9 [7]. The subsequent reduction to aminoacetonitrile 10 [8] followed by reaction with S2Cl2 was expected to give the desired thiadiazole 5 [9].
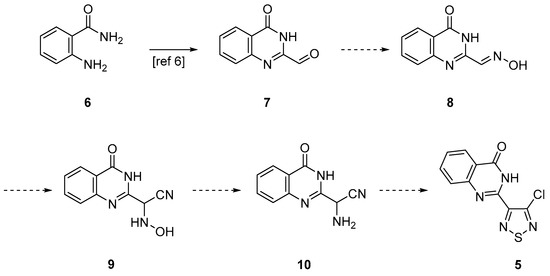
Scheme 2.
Proposed synthesis for thiadiazole 5.
The reaction of quinazolinone-2-carbaldehyde 7 with hydroxylamine hydrochloride (1.1 equiv), in the presence of K2CO3 (1 equiv), in EtOH, at ca. 60 °C led after 3 h to complete consumption of the starting aldehyde and on work-up isolation of oxime 8 in 58% yield (Scheme 3).
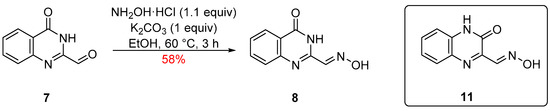
Scheme 3.
Synthesis of (E)-4-oxo-3,4-dihydroquinazoline-2-carbaldehyde oxime (8) and structure of the isomeric (E)-3-oxo-3,4-dihydroquinoxaline-2-carbaldehyde oxime (11).
Product 8 was isolated as colorless needles, mp 237–238 °C (from EtOH/H2O). FTIR spectroscopy showed an oxime ν(O-H) stretch at 3280 cm−1 along with an amide ν(N-H) stretch at 3173 cm−1, an oxime ν(C-H) stretch at 2876 cm−1 and a broad ν(C=O) stretch at 1678 cm−1. Meanwhile, mass spectrometry revealed a molecular ion (MH+) peak of m/z 190, agreeing with the addition of NH2OH and loss of H2O from the starting aldehyde 7. 13C NMR spectroscopy showed the presence of five CH resonances and four quaternary carbon resonances (see Supplementary Materials for the complete spectra). At the same time, a correct elemental analysis (CHN) was obtained for the molecular formula C9H7N3O2, agreeing with the structure shown above. Structural support was also provided by single-crystal X-ray diffraction studies (Figure 2). To the best of our knowledge, compound 8 has not been reported in the literature and could have many potential uses. Importantly, the structurally similar isomer quinoxalin-2(1H)-one-3-carbaldoxime (11) (Scheme 3) has been used as a scaffold for the preparation of benzimidazoles [10]. At the same time, other analogs were investigated as neurologically active compounds for the treatment of Alzheimer’s disease [11] or as ligands to ruthenium and osmium complexes with anticancer properties [12].
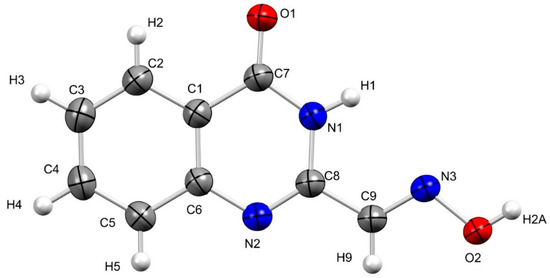
Figure 2.
Geometry of (E)-4-oxo-3,4-dihydroquinazoline-2-carbaldehyde oxime (8) in the crystal; crystallographic atom numbering. Thermal ellipsoids at 50% probability.
X-ray crystallography indicated that quinazoline 8 is planar in the crystalline state and forms sheets (intersheet distance is 4.03 Å) of off-set dimers held together by hydrogen bond interactions [d(O-H…O) ~2.60 Å, Θ(O-H…O) 173.41°] (Figure 3). Notable intramolecular bond lengths include the C7-O1 and N2-C8 bond lengths typical of double bonds; d(C7=O1) = 1.23 Å and d(C8=N2) = 1.29 Å, respectively.
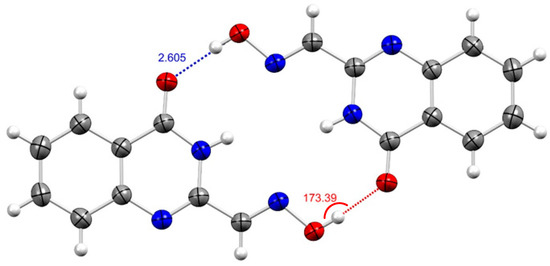
Figure 3.
ORTEP view of notable intermolecular interactions (Å, in blue) and related bond angles (°, in red) from the crystal structure of (E)-4-oxo-3,4-dihydroquinazoline-2-carbaldehyde oxime (8); thermal ellipsoids at 50% probability.
After the synthesis of oxime 8, the addition of cyanide was attempted, but unfortunately, no reaction occurred with KCN (10 equiv), in dry DMF at ca. 20 °C for 2 d, as well as, with 18-crown-6 (1 equiv) at ca. 100 °C for 3 d. This reaction will be further investigated in the future.
3. Materials and Methods
The reaction mixture was monitored by TLC using commercial glass-backed thin layer chromatography (TLC) plates (Merck Kieselgel 60 F254, Darmstadt, Germany). The plates were observed under UV light at 254 and 365 nm. The melting point was determined using a PolyTherm-A, Wagner & Munz, Kofler–Hotstage Microscope apparatus (Wagner & Munz, Munich, Germany). The solvent used for recrystallization is indicated after the melting point. The UV-vis spectrum was obtained using a Perkin-Elmer Lambda-25 UV-vis spectrophotometer (Perkin-Elmer, Waltham, MA, USA) and inflections are identified by the abbreviation “inf”. The IR spectrum was recorded on a Shimadzu FTIR-NIR Prestige-21 spectrometer (Shimadzu, Kyoto, Japan) with Pike Miracle Ge ATR accessory (Pike Miracle, Madison, WI, USA), and strong, medium, and weak peaks were represented by s, m, and w, respectively. 1H and 13C NMR spectra were recorded on a Bruker Avance 500 machine [at 500 and 125 MHz, respectively, (Bruker, Billerica, MA, USA)]. Deuterated solvents were used for homonuclear lock, and the signals are referenced to the deuterated solvent peaks. Attached proton test (APT) NMR studies were used to assign the 13C peaks as CH3, CH2, CH, and Cq (quaternary). The Matrix-Assisted Laser Desorption/Ionization-Time Of Flight (MALDI-TOF) mass spectrum (+ve mode) was recorded on a Bruker Autoflex III Smartbeam instrument (Bruker). The elemental analysis was run by the London Metropolitan University Elemental Analysis Service. 4-Oxo-3,4-dihydroquinazoline-2-carbaldehyde (7) was prepared according to the literature procedure [6].
(E)-4-Oxo-3,4-dihydroquinazoline-2-carbaldehyde oxime (8). To a stirred mixture of 4-oxo-3,4-dihydroquinazoline-2-carbaldehyde (7) (87 mg, 0.50 mmol) in EtOH (2 mL) at ca. 20 °C was added NH2OH·HCl (38 mg, 0.55 mmol) followed by K2CO3 (69 mg, 0.50 mmol) and the mixture was then warmed to ca. 60 °C and stirred at this temperature until complete consumption of the starting material (TLC, 3 h). The mixture was then cooled to ca. 20 °C and then H2O (5 mL) was added. The red precipitate formed was filtered, washed with H2O (5 mL) and dried in vacuo to give the title compound 8 (55 mg, 58%) as colorless needles, mp 237–238 °C (from EtOH/H2O); Rf 0.25 (DCM/t-BuOMe 90:10); (found: C, 57.21; H, 3.69; N, 22.21. C9H7N3O2 requires C, 57.14; H, 3.73; N, 22.21%); λmax(THF)/nm 306 inf (log ε 3.98), 316 (4.04), 346 (3.80), 364 inf (3,50); vmax/cm−1 3281 w (O-H), 3173 w (N-H), 3065 w and 3003 w (C-H arom), 2876 w and 2799 w (oxime C-H), 1678 s (C=O), 1599 m, 1564 w, 1510 w, 1470 m, 1344 m, 1275 w, 1260 w, 1142 w, 1038 m, 1020 m, 1003 m, 922 w, 878 m, 808 m, 781 m, 748 m, 733 m; δH(500 MHz; DMSO-d6) 12.43 (1H, br. s, OH), 12.01 (1H, br. s, NH), 8.13 (1H, d, J = 7.6, Ar CH), 7.87 (1H, s, NCH), 7.83 (1H, dd, J = 7.4, 7.4, Ar CH), 7.69 (1H, d, J = 8.2, Ar CH), 7.54 (1H, dd, J = 7.4, 7.4, Ar CH); δC(125 MHz; DMSO-d6) 160.9 (Cq), 148.2 (Cq), 148.1 (Cq), 143.4 (CH), 134.7 (CH), 127.4 (CH), 127.3 (CH), 126.0 (CH), 122.0 (Cq); m/z (MALDI-TOF) 228 (M + K+, 100%), 212 (M + Na+, 82), 190 (MH+, 93), 113 (35).
X-ray crystallographic studies on (E)-4-oxo-3,4-dihydroquinazoline-2-carbaldehyde oxime (8). Data were collected on an Oxford-Diffraction Supernova diffractometer, equipped with a CCD area detector utilizing Cu-Kα radiation (λ = 1.5418 Å). A suitable crystal was attached to glass fibers using paratone-N oil and transferred to a goniostat, where they were cooled for data collection. Unit cell dimensions were determined and refined using 6738 (4.485° ≤ θ ≤ 77.063°) reflections. Empirical absorption corrections (multi-scan based on symmetry-related measurements) were applied using CrysAlis RED software.17 The structures were solved by direct method and refined on F2 using full-matrix least-squares using SHELXL97.18 Software packages used: CrysAlis CCD17 for data collection, CrysAlis RED17 for cell refinement and data reduction, WINGX for geometric calculations,19 and DIAMOND20 for molecular graphics. The non-H atoms were treated anisotropically. The hydrogen atoms were placed in calculated, ideal positions and refined as riding on their respective carbon atoms.
Crystal refinement data for (E)-4-oxo-3,4-dihydroquinazoline-2-carbaldehyde oxime (8): isolated as colorless needles (from EtOH/H2O vapor diffusion), C9H7N3O2, M = 189.18, Monoclinic, space group P21/c, a = 10.0944(12) Å, b = 9.8586(8) Å, c = 9.2424(10) Å, α = 90°, β = 11.696 (13)°, γ = 90°, V = 854.61(17) Å3, Z = 4, T = 100(2) K, ρcalcd = 0.908 g·cm−3, θmax = 77.063°. Refinement of 128 parameters on 1780 independent reflections out of 6738 measured reflections (Rint = 0.0789) led to R1 = 0.1182 [I > 2σ(I)], wR2 = 0.3004 (all data), and S = 1.087 with the largest difference peak and hole of 0.581 and −0.326 e·Å−3, respectively. (CCDC: 2083591).
Supplementary Materials
Supplementary materials are available online. Mol file, 1H and 13C NMR spectra, and IR spectrum.
Author Contributions
P.A.K. and A.S.K. conceived the experiments; A.S.K. designed and performed the experiments, analyzed the data, and wrote the paper; A.K. collected the X-ray crystallography data. All authors have read and agreed to the published version of the manuscript.
Funding
The Cyprus Research Promotion Foundation funded this research, grant numbers ΣΤΡAΤHΙΙ/0308/06, NEKYP/0308/02 ΥΓΕΙA/0506/19 and ΕΝΙΣΧ/0308/83.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the following organizations and companies in Cyprus for generous donations of chemicals and glassware: the State General Laboratory, the Agricultural Research Institute, the Ministry of Agriculture, MedoChemie Ltd., Medisell Ltd., and Biotronics Ltd. Furthermore, we thank the A.G. Leventis Foundation for helping to establish the NMR facility at the University of Cyprus.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Rewcastle, G.W. Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Chapter 8.02; Volume 8, pp. 117–272. [Google Scholar]
- Kalogirou, A.S.; Kourtellaris, A.; Koutentis, P.A. Synthesis and Reactivity of 3′,5′-Dichloro-1H-spiro(quinazoline-2,4′-[1,2,6]thiadiazin)-4(3H)-ones. Eur. J. Org. Chem. 2019, 5462–5474. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Kourtellaris, A.; Koutentis, P.A. Synthesis of 2-Cyanoquinazolin-4-ones from 3′,5′-Dichloro-1H-spiro(quinazoline-2,4′-[1,2,6]thiadiazin)-4(3H)-ones. Chem. Select 2020, 5, 1884–1889. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Kourtellaris, A.; Koutentis, P.A. Synthesis and chemistry of benzo[e][1,2,6]thiadiazino[3,4-b][1,4]diazepin-10(11H)-ones and related ring transformations. J. Org. Chem. 2021, 86, 5702–5713. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Peng, B.; Xiao, B.B.; Cao, S.L.; Yang, C.R.; Wang, W.Z.; Wang, F.C.; Li, H.-Y.; Yuan, X.-L.; Shi, R.; et al. Synthesis and evaluation of chalcone analogues containing a 4-oxoquinazolin-2-yl group as potential anti-tumor agents. Eur. J. Med. Chem. 2019, 162, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, L.; Hartung, W.H. α-Hydroxylamino Nitriles and α-Hydroxylamino Acids. J. Org. Chem. 1958, 23, 964–967. [Google Scholar] [CrossRef]
- Jin, C.; Burgess, J.P.; Kepler, J.A.; Cook, C.E. Copper-Catalyzed Cyclization of Steroidal Acylaminoacetylenes: Syntheses of Novel 11β-Aryl-17,17-spiro[(4′H,5′-methylene)oxazol]-Substituted Steroids. Org. Lett. 2007, 9, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Maspero, M.; Volpato, D.; Cirillo, D.; Chen, N.Y.; Messerer, R.; Sotriffer, C.; De Amici, M.; Holzgrabe, U.; Dallanoce, C. Tacrine-xanomeline and tacrine-iperoxo hybrid ligands: Synthesis and biological evaluation at acetylcholinesterase and M1 muscarinic acetylcholine receptors. Bioorg. Chem. 2020, 96, 103633–103646. [Google Scholar] [CrossRef] [PubMed]
- Mamedov, V.A.; Zhukova, N.A.; Syakaev, V.V.; Gubaidullin, A.T.; Kadyrova, M.S.; Beschastnova, T.N.; Rizvanov, I.K.; Latypov, S.K. Simultaneous formation of 3-(benzimidazol-2-yl)quinoxalin-2(1H)-ones and 2-(benzimidazol-2-yl)quinoxalines from quinoxalin-2(1H)-one-3-carbaldoximes when exposed to 1,2-benzenediamines. Tetrahedron 2020, 76, 131721. [Google Scholar] [CrossRef]
- Kok, G.B.; Leung, B.K.Y.; Gautier, E.C.L.; Barnam, K.J. Neurologically Active Compounds. WO Patent 031161, 15 April 2004. [Google Scholar]
- Ginzinger, W.; Muhlgassner, G.; Arion, V.B.; Jakupec, M.A.; Roller, A.; Galanski, M.; Reithofer, M.; Berger, W.; Keppler, B.K. A SAR study of novel antiproliferative ruthenium and osmium complexes with quinoxalinone ligands in human cancer cell lines. J. Med. Chem. 2012, 55, 3398–3413. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).