Ethyl 1-Butyl-2-(2-hydroxy-4-methoxyphenyl)-1H-benzo[d]imidazole-5-carboxylate
Abstract
1. Introduction
2. Results
3. Discussion
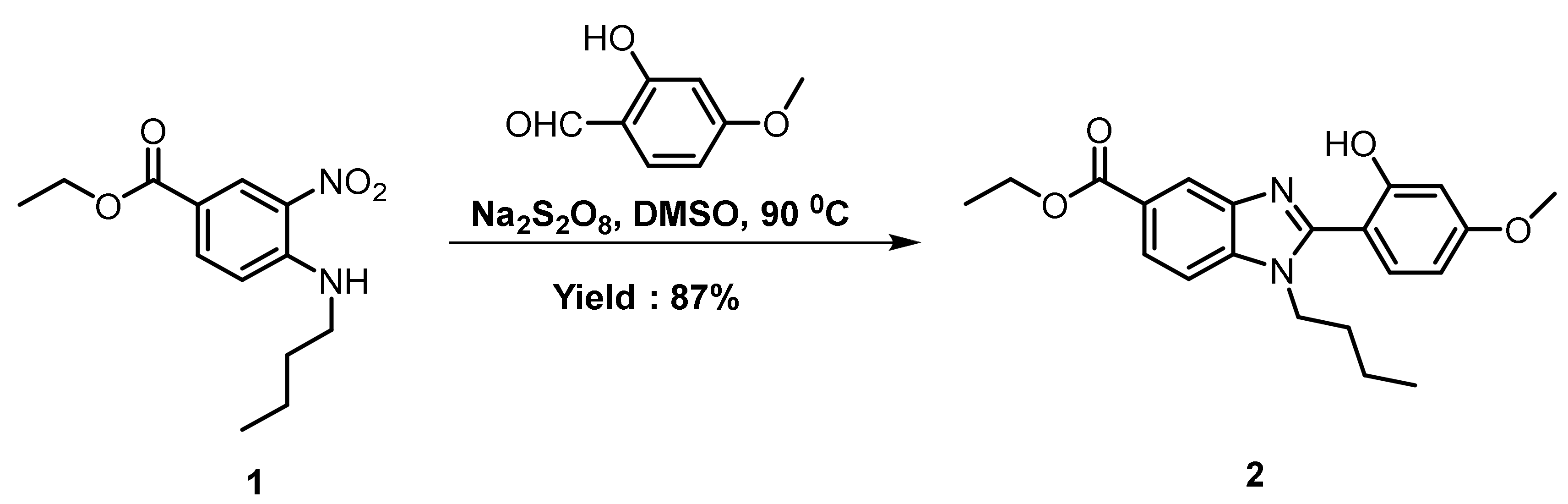
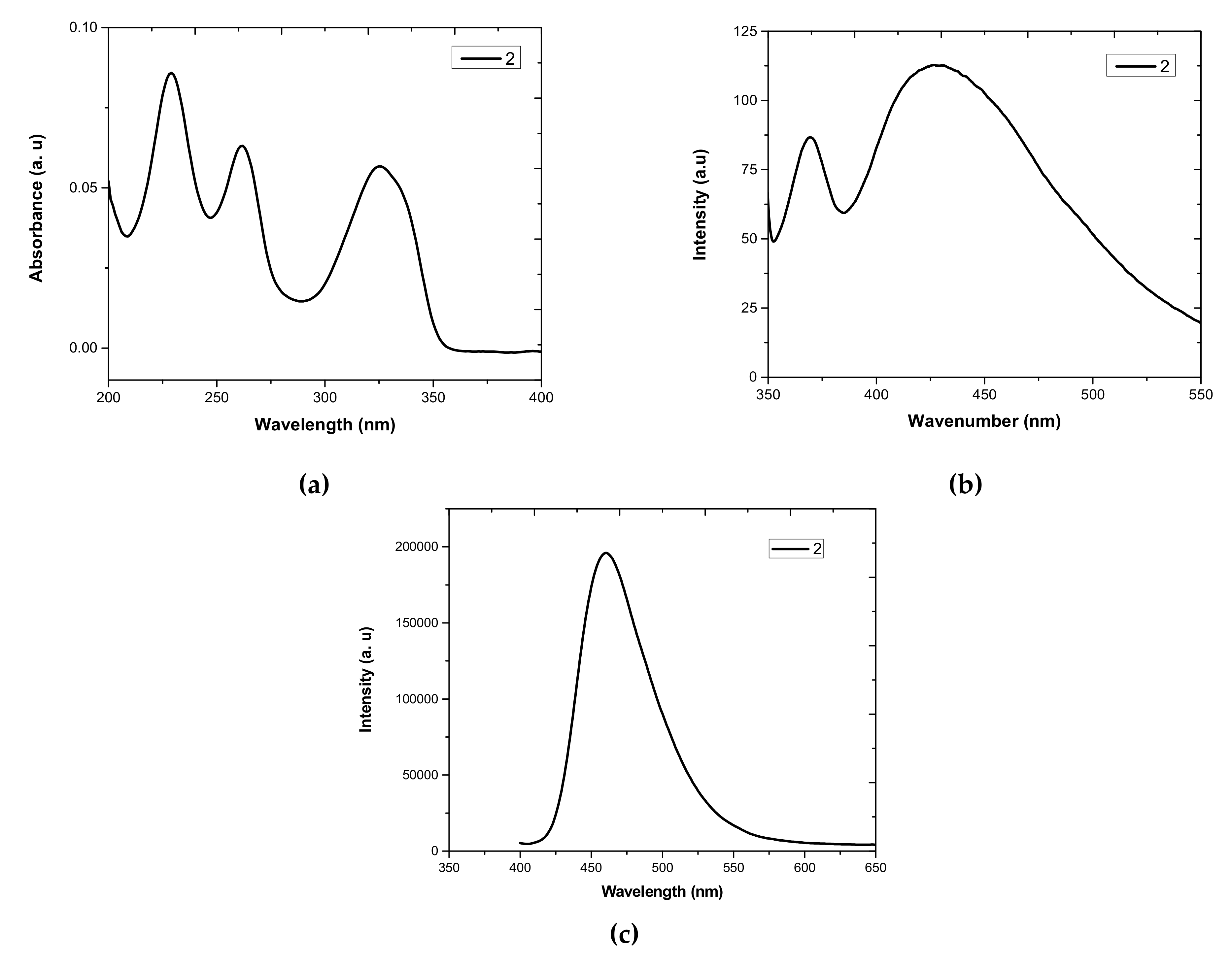
3.1. UV-Vis Absorption and Photoluminescence (PL) Properties
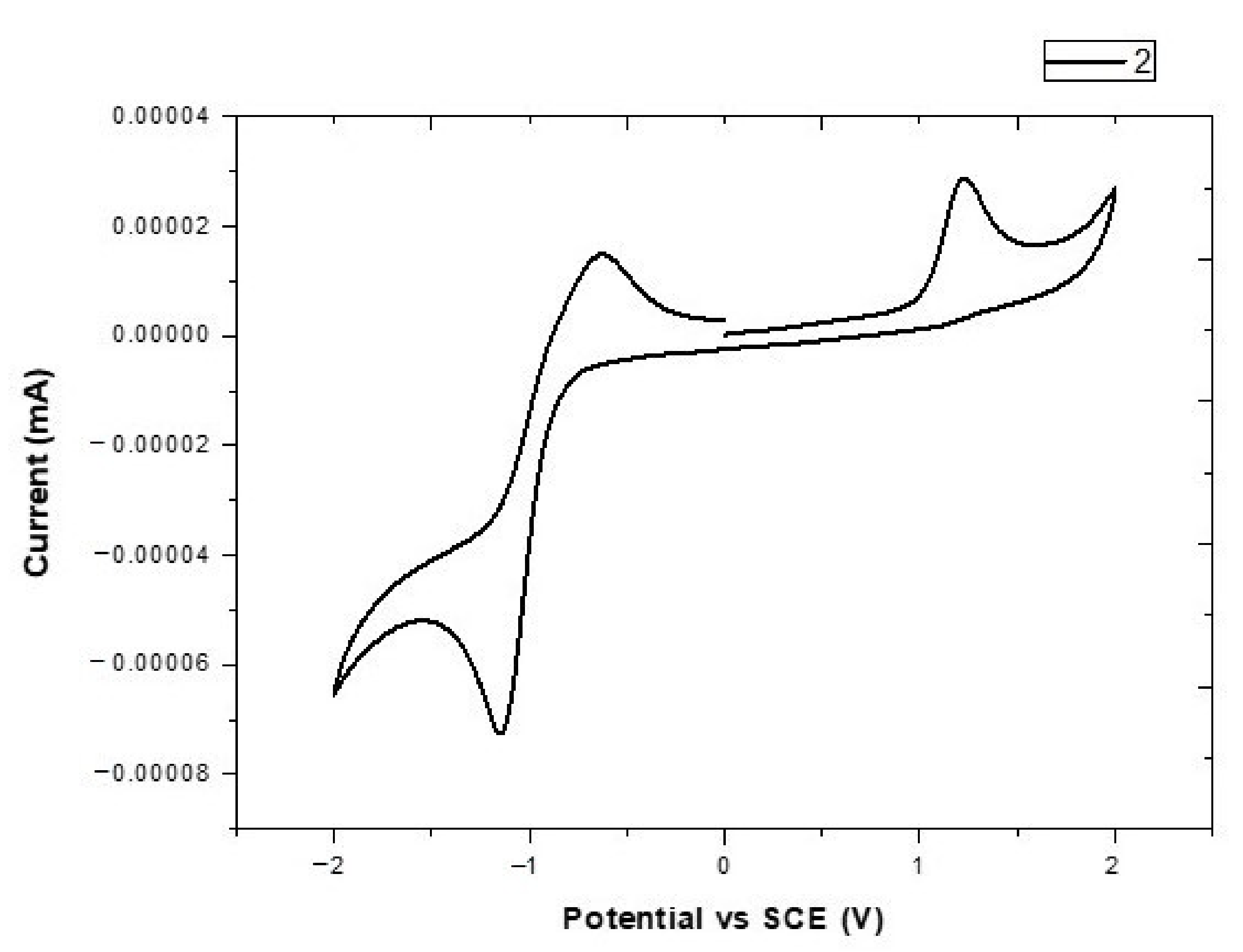
3.2. Electrochemical Properties
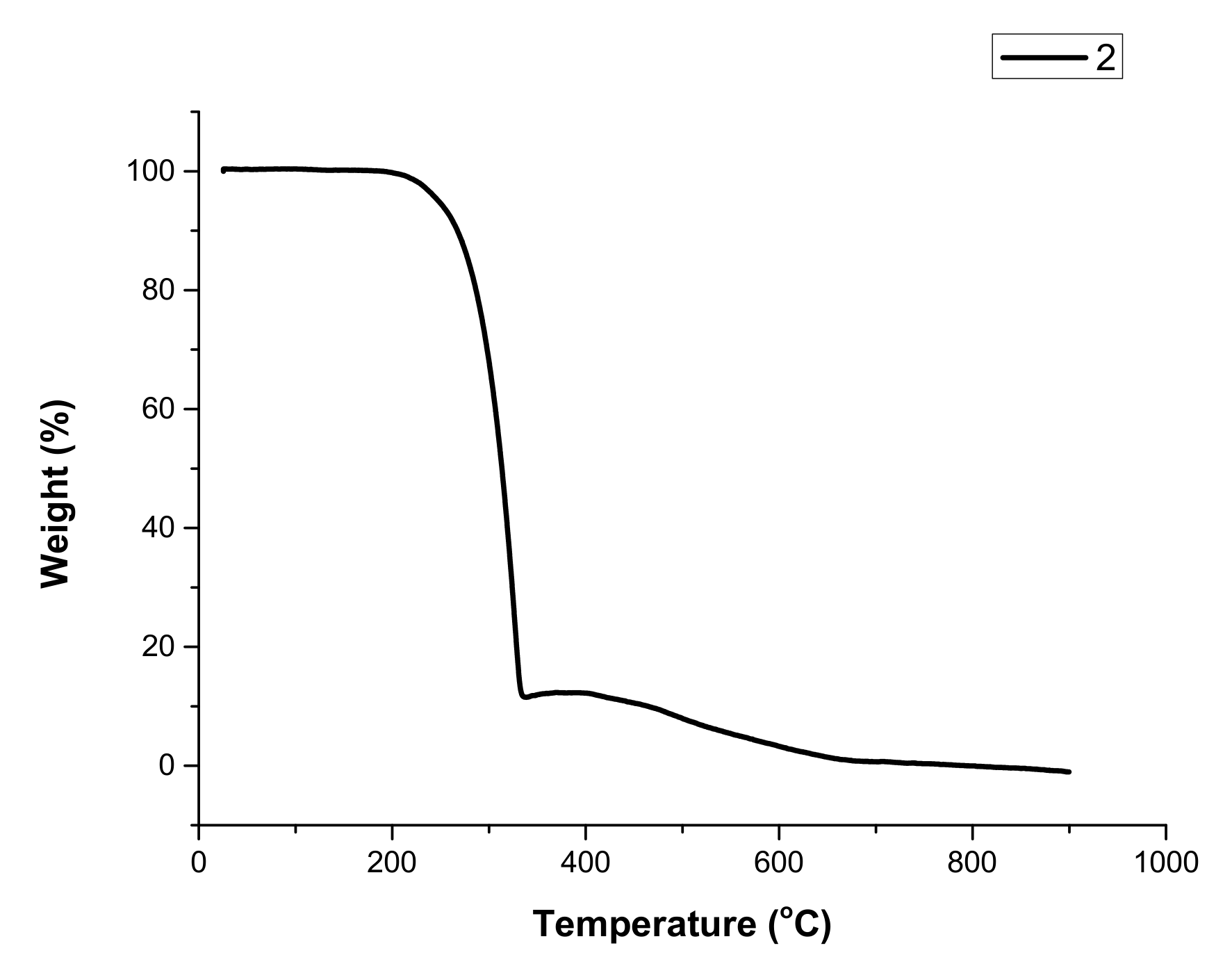
3.3. Thermal Properties
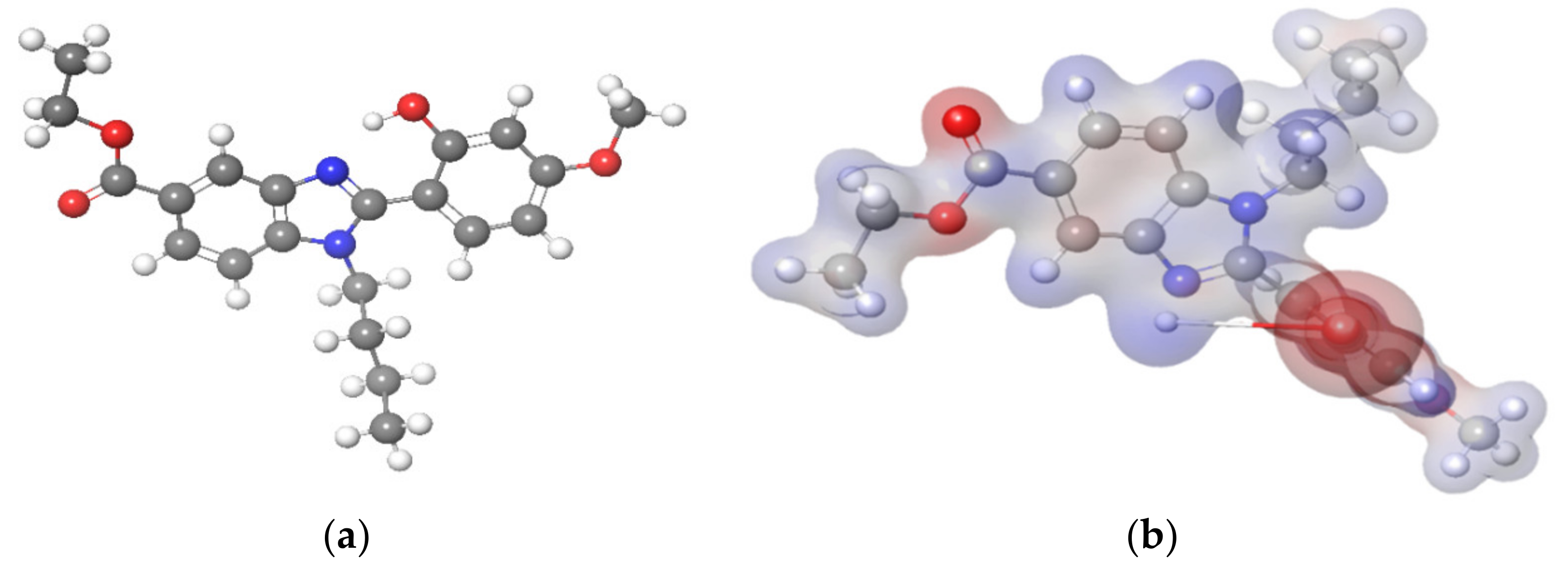
3.4. Molecular Electrostatic Potential (MEP)
4. Materials and Methods
Ethyl 1-butyl-2-(2-hydroxy-4-methoxyphenyl)-1H-benzo[d]imidazole-5-carboxylate (2)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Paramashivappa, R.; Kumar, P.P.; Rao, P.S.; Rao, A.S. Design, synthesis and biological evaluation of benzimidazole/benzothiazole and benzoxazole derivatives as cyclooxygenase inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 657–660. [Google Scholar] [CrossRef]
- Achar, K.C.; Hosamani, K.M.; Seetharamareddy, H.R. In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur. J. Med. Chem. 2010, 5, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Solomon, V.R.; Lee, H.; Trivedi, P. Synthesis and biological evaluation of 2-(phenyl)-3H-benzo [d] imidazole-5-carboxylic acids and its methyl esters as potent anti-breast cancer agents. Arab. J. Chem. 2017, 10, S1788–S1794. [Google Scholar] [CrossRef]
- Kwak, H.J.; Pyun, Y.M.; Kim, J.Y.; Pagire, H.S.; Kim, K.Y.; Kim, K.R.; Dal Rhee, S.; Jung, W.H.; Song, J.S.; Bae, M.A.; et al. Synthesis and biological evaluation of aminobenzimidazole derivatives with a phenylcyclohexyl acetic acid group as anti-obesity and anti-diabetic agents. Bioorg. Med. Chem. Lett. 2013, 23, 4713–4718. [Google Scholar] [CrossRef]
- Klimesova, V.; Koci, J.; Pour, M.; Stachel, J.; Waisser, K.; Kaustova, J. Synthesis and preliminary evaluation of benzimidazole derivatives as antimicrobial agents. Eur. J. Med. Chem. 2002, 37, 409–418. [Google Scholar] [CrossRef]
- Kus, C.; Ayhan-Kilcigil, G.; Ozbey, S.; Kaynak, F.B.; Kaya, M.; Çoban, T.; Can-Eke, B. Synthesis and antioxidant properties of novel N-methyl-1, 3, 4-thiadiazol-2-amine and 4-methyl-2H-1, 2, 4-triazole-3 (4H)-thione derivatives of benzimidazole class. Bioorg. Med. Chem. 2008, 16, 4294–4303. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.; Audhya, A.; Abtab, S.M.T.; Ghosh, S.; Tiekink, E.R.; Chaudhury, M. Anion-controlled assembly of silver (I) complexes of multiring heterocyclic ligands: A structural and photophysical study. Cryst. Growth Des. 2010, 10, 1269–1282. [Google Scholar] [CrossRef]
- Huang, W.K.; Wu, H.P.; Lin, P.L.; Lee, Y.P.; Diau, E.W.G. Design and Characterization of Heteroleptic Ruthenium Complexes Containing Benzimidazole Ligands for Dye-Sensitized Solar Cells: The Effect of Fluorine Substituents on Photovoltaic Performance. J. Phys. Chem. Lett. 2012, 3, 1830–1835. [Google Scholar] [CrossRef]
- Vijayan, N.; Balamurugan, N.; Babu, R.R.; Gopalakrishnan, R.; Ramasamy, P. Growth and characterization studies of organic NLO crystals of benzimidazole by melt technique. J. Cryst. Growth. 2005, 275, 1895–1900. [Google Scholar] [CrossRef]
- Singh, N.; Jang, D.O. Benzimidazole-based tripodal receptor: Highly selective fluorescent chemosensor for iodide in aqueous solution. Org. Lett. 2007, 9, 1991–1994. [Google Scholar] [CrossRef]
- Wu, Y.C.; You, J.Y.; Jiang, K.; Wu, H.Q.; Xiong, J.F.; Wang, Z.Y. Novel benzimidazole-based ratiometric fluorescent probes for acidic pH. Dyes Pigm. 2018, 149, 1–7. [Google Scholar] [CrossRef]
- Behera, S.K.; Sadhuragiri, G.; Elumalai, P.; Sathiyendiran, M.; Krishnamoorthy, G. Exclusive excited state intramolecular proton transfer from a 2-(2′-hydroxyphenyl) benzimidazole derivative. RSC Adv. 2016, 6, 59708–59717. [Google Scholar] [CrossRef]
- Douhal, A.; Amat-Guerri, F.; Lillo, M.P.; Acuna, A.U. Proton transfer spectroscopy of 2-(2’-hydroxyphenyl) imidazole and 2-(2’-hydroxyphenyl) benzimidazole dyes. J. Photoch. Photobio. A 1994, 78, 127–138. [Google Scholar] [CrossRef]
- Padalkar, V.S.; Ramasami, P.; Sekar, N. A combined experimental and DFT-TDDFT study of the excited-state intramolecular proton transfer (ESIPT) of 2-(2′-hydroxyphenyl) imidazole derivatives. J. Fluoresc. 2013, 23, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Sontakke, V.A.; Kate, A.N.; Ghosh, S.; More, P.; Gonnade, R.; Kumbhar, N.M.; Kumbhar, A.A.; Chopade, B.A.; Shinde, V.S. Synthesis, DNA interaction and anticancer activity of 2-anthryl substituted benzimidazole derivatives. New J. Chem. 2015, 39, 4882–4890. [Google Scholar] [CrossRef]
- Ma, S.; Fu, Y.; Ni, D.; Mao, J.; Xie, Z.; Tu, G. Spiro-fluorene based 3D donor towards efficient organic photovoltaics. Chem. Commun. 2012, 48, 1187–11849. [Google Scholar] [CrossRef]
- Nie, J.; Li, N.; Ni, Z.; Zhao, Y.; Zhang, L. A sensitive tetraphenylethene-based fluorescent probe for Zn2+ ion involving ESIPT and CHEF processes. Tetrahedron Lett. 2017, 58, 1980–1984. [Google Scholar] [CrossRef]
- Yuen, J.D.; Fan, J.; Seifter, J.; Lim, B.; Hufschmid, R.; Heeger, A.J.; Wudl, F. High performance weak donor–acceptor polymers in thin film transistors: Effect of the acceptor on electronic properties, ambipolar conductivity, mobility, and thermal stability. J. Am. Chem. Soc. 2011, 133, 20799–20807. [Google Scholar] [CrossRef] [PubMed]
- Gorrn, P.; Lehnhardt, M.; Kowalsky, W.; Riedl, T.; Wagner, S. Elastically Tunable Self-Organized Organic Lasers. Adv. Mater. 2011, 23, 869–872. [Google Scholar] [CrossRef]
- Qin, W.; Lam, J.W.; Yang, Z.; Chen, S.; Liang, G.; Zhao, W.; Kwok, H.S.; Tang, B.Z. Red emissive AIE luminogens with high hole-transporting properties for efficient non-doped OLEDs. Chem. Commun. 2015, 51, 7321–7324. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, N.; Dong, Y.; Tang, R.; Lu, P.; Cai, P.; Li, Q.; Ma, D.; Qin, J.; Li, Z. Similar or totally different: The control of conjugation degree through minor structural modifications, and deep-blue aggregation-induced emission luminogens for non-doped OLEDs. Adv. Funct. 2013, 23, 2329–2337. [Google Scholar] [CrossRef]
- Chen, L.; Lin, G.; Peng, H.; Nie, H.; Zhuang, Z.; Shen, P.; Ding, S.; Huang, D.; Hu, R.; Chen, S.; et al. Dimesitylboryl-functionalized tetraphenylethene derivatives: Efficient solid-state luminescent materials with enhanced electron-transporting ability for nondoped OLEDs. J. Mater. Chem. C. 2016, 4, 5241–5247. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, R.; Zhao, Y.; Ni, Z. A new series of N-substituted tetraphenylethene-based benzimidazoles: Aggregation-induced emission, fast-reversible mechanochromism and blue electroluminescence. Dyes Pigm. 2018, 148, 276–285. [Google Scholar] [CrossRef]
- Vasantha, K.; Basavarajaswamy, G.; Rai, M.V.; Boja, P.; Pai, V.R.; Shruthi, N.; Bhat, M. Rapid ‘one-pot’synthesis of a novel benzimidazole-5-carboxylate and its hydrazone derivatives as potential anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. Lett. 2015, 25, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.D.; Hougland, J.L.; Markarian, S.A. Formation and Anomalous Behavior of Aminonaphthalene−Cinnamonitrile Exciplexes. J. Phys. Chem. A 2000, 104, 3261–3268. [Google Scholar] [CrossRef]
- Tamer., O.; Tamer, S.A.; Idil, O.; Avci, D.; Vural, H.; Atalay, Y. Antimicrobial activities, DNA interactions, spectroscopic (FT-IR and UV-Vis) characterizations, and DFT calculations for pyridine-2-carboxylic acid and its derivates. J. Mol. Struct. 2018, 1152, 399–408. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathyanarayana, R.; Poojary, B. Ethyl 1-Butyl-2-(2-hydroxy-4-methoxyphenyl)-1H-benzo[d]imidazole-5-carboxylate. Molbank 2021, 2021, M1192. https://doi.org/10.3390/M1192
Sathyanarayana R, Poojary B. Ethyl 1-Butyl-2-(2-hydroxy-4-methoxyphenyl)-1H-benzo[d]imidazole-5-carboxylate. Molbank. 2021; 2021(1):M1192. https://doi.org/10.3390/M1192
Chicago/Turabian StyleSathyanarayana, Reshma, and Boja Poojary. 2021. "Ethyl 1-Butyl-2-(2-hydroxy-4-methoxyphenyl)-1H-benzo[d]imidazole-5-carboxylate" Molbank 2021, no. 1: M1192. https://doi.org/10.3390/M1192
APA StyleSathyanarayana, R., & Poojary, B. (2021). Ethyl 1-Butyl-2-(2-hydroxy-4-methoxyphenyl)-1H-benzo[d]imidazole-5-carboxylate. Molbank, 2021(1), M1192. https://doi.org/10.3390/M1192




