1,1’,1’’-(2’,4’-Dinitro-[1,1’-biphenyl]-2,4,6-triyl)tripiperidine
Abstract
:1. Introduction
2. Results
3. Materials and Methods
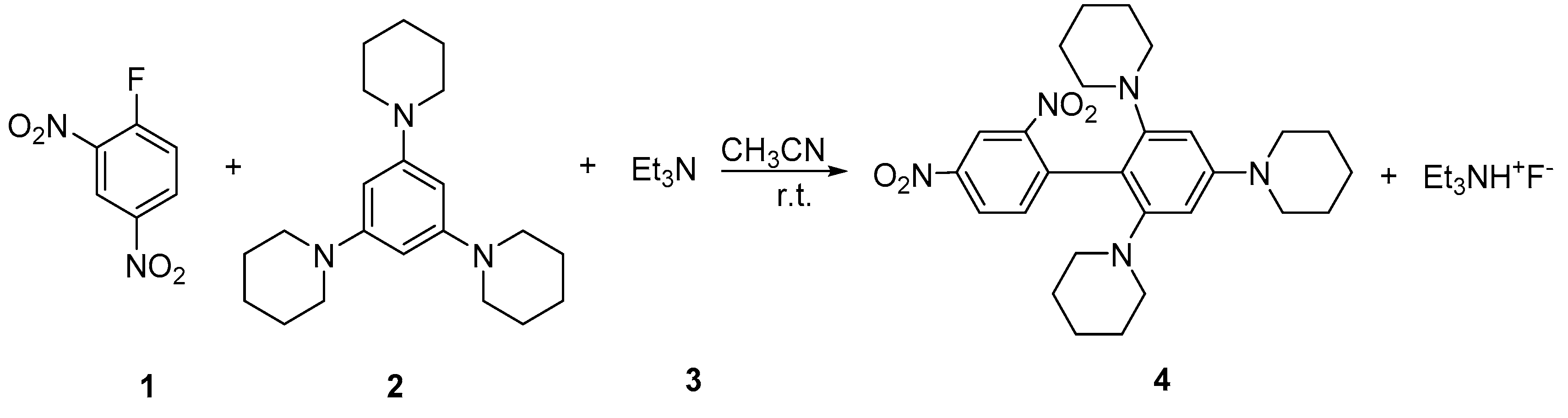
Synthesis of 1,1’,1’’-(2’,4’-Dinitro-[1,1’-biphenyl]-2,4,6-triyl)tripiperidine
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Terrier, F. Modern Nucleophilic Aromatic Substitution; Wiley VCH: Weinheim, Germany, 2013. [Google Scholar]
- Terrier, F. Nucleophilic Aromatic Displacement; VCH: New York, NY, USA, 1991. [Google Scholar]
- Ouellette, R.J.; Rawn, J.D. Electrophilic aromatic substitution. In Organic Chemistry: Structure, Mechanism and Synthesis, 1st ed.; Elsevier: San Diego, CA, USA, 2014; Chapter 13; pp. 417–451. [Google Scholar]
- Ouellette, R.J.; Rawn, J.D. Electrophilic aromatic substitution. In Organic Chemistry: Structure, Mechanism and Synthesis, 2nd ed.; Elsevier: San Diego, CA, USA, 2018; Chapter 13; pp. 375–407. [Google Scholar]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- He, G.S.; Tan, L.S.; Zheng, Q.; Prasad, P.N. Multiphoton absorbing materials: Molecular designs, characterizations, and applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef]
- Boga, C.; Micheletti, G.; Cino, S.; Fazzini, S.; Forlani, L.; Zanna, N.; Spinelli, D. C–C coupling between trinitrothiophenes and triaminobenzenes: Zwitterionic intermediates and new all-conjugated structures. Org. Biomol. Chem. 2016, 14, 4267–4275. [Google Scholar] [CrossRef]
- Micheletti, G.; Boga, C.; Pafundi, M.; Pollicino, S.; Zanna, N. New electron-donor and -acceptor architectures from benzofurazans and sym-triaminobenzenes: Intermediates, products and an unusual nitro group shift. Org. Biomol. Chem. 2016, 14, 768–776. [Google Scholar] [CrossRef]
- Boga, C.; Cino, S.; Micheletti, G.; Padovan, D.; Prati, L.; Mazzanti, A.; Zanna, N. New azo-decorated N-pyrrolidinylthiazoles: Synthesis, properties and an unexpected remote substituent effect transmission. Org. Biomol. Chem. 2016, 14, 7061–7068. [Google Scholar] [CrossRef]
- Del Vecchio, E.; Boga, C.; Forlani, L.; Tozzi, S.; Micheletti, G.; Cino, S. Ring closure of azo compounds to 1,2-annulated benzimidazole derivatives and further evidence of reversibility of the azo-coupling reaction. J. Org. Chem. 2015, 80, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Boga, C.; Del Vecchio, E.; Tozzi, S.; Forlani, L.; Monari, M.; Micheletti, G.; Zanna, N. First isolation of a Wheland intermediate in the azo-coupling reaction, its X-ray crystal structure determination and products from its evolution. ARKIVOC 2014, iv, 51–66. [Google Scholar]
- Chassot, L.; Braun, H.-J. Colouring Agents for Keratin Fibres Containing (1.1-biphenyl)-2,4-diamine Derivatives in Addition to Novel (1.1-biphenyl)-2,4-diamine-derivatives. U.S. Patent 2003/0172470 A1, 18 September 2003. [Google Scholar]
- Mąkosza, M. How does nucleophilic aromatic substitution in nitroarenes really proceed: General mechanism. Synlett 2017, 49, 3247–3254. [Google Scholar] [CrossRef]
- Błaziak, K.; Danikiewicz, W.; Mąkosza, M. How does nucleophilic aromatic substitution really proceed in nitroarenes? Computational prediction and experimental verification. J. Am. Chem. Soc. 2016, 138, 7276–7281. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, F.; Agster, W.; Fischer, P.; Jogun, K.H.; Stezowski, J.J.; Daltrozzo, E.; Kollmannsberger-Von Nell, G. Synthesis, structure, and spectral behaviour of donor-acceptor substituted biphenyls. J. Org. Chem. 1983, 48, 4649–4658. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheletti, G.; Telese, D.; Fazzini, S.; Boga, C. 1,1’,1’’-(2’,4’-Dinitro-[1,1’-biphenyl]-2,4,6-triyl)tripiperidine. Molbank 2020, 2020, M1154. https://doi.org/10.3390/M1154
Micheletti G, Telese D, Fazzini S, Boga C. 1,1’,1’’-(2’,4’-Dinitro-[1,1’-biphenyl]-2,4,6-triyl)tripiperidine. Molbank. 2020; 2020(3):M1154. https://doi.org/10.3390/M1154
Chicago/Turabian StyleMicheletti, Gabriele, Dario Telese, Silvia Fazzini, and Carla Boga. 2020. "1,1’,1’’-(2’,4’-Dinitro-[1,1’-biphenyl]-2,4,6-triyl)tripiperidine" Molbank 2020, no. 3: M1154. https://doi.org/10.3390/M1154
APA StyleMicheletti, G., Telese, D., Fazzini, S., & Boga, C. (2020). 1,1’,1’’-(2’,4’-Dinitro-[1,1’-biphenyl]-2,4,6-triyl)tripiperidine. Molbank, 2020(3), M1154. https://doi.org/10.3390/M1154





