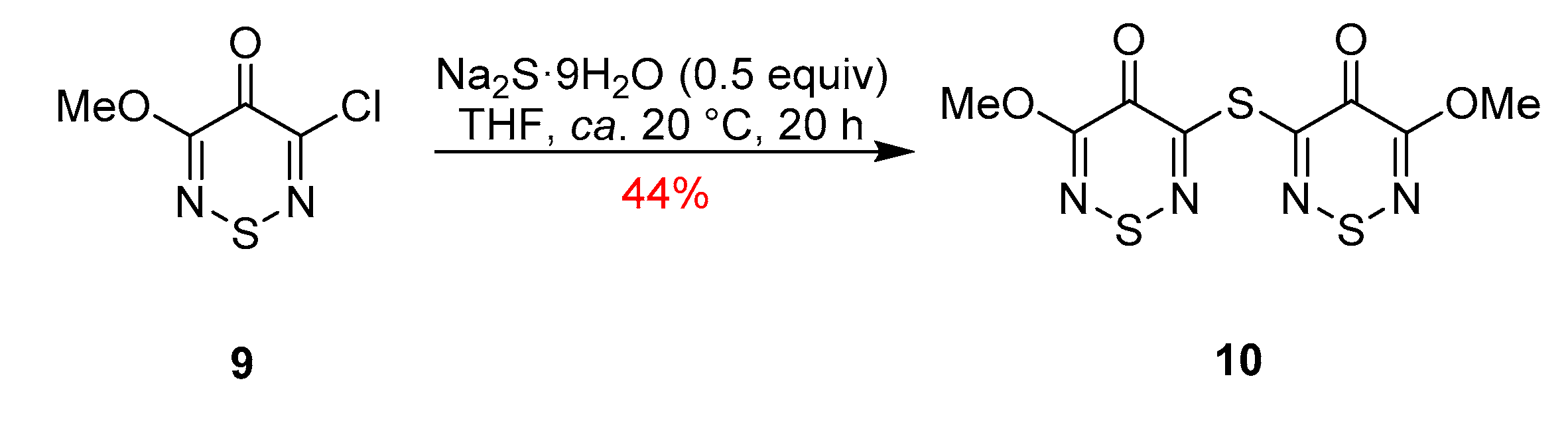
5,5′-Thiobis(3-methoxy-4H-1,2,6-thiadiazin-4-one)
Abstract
:1. Introduction
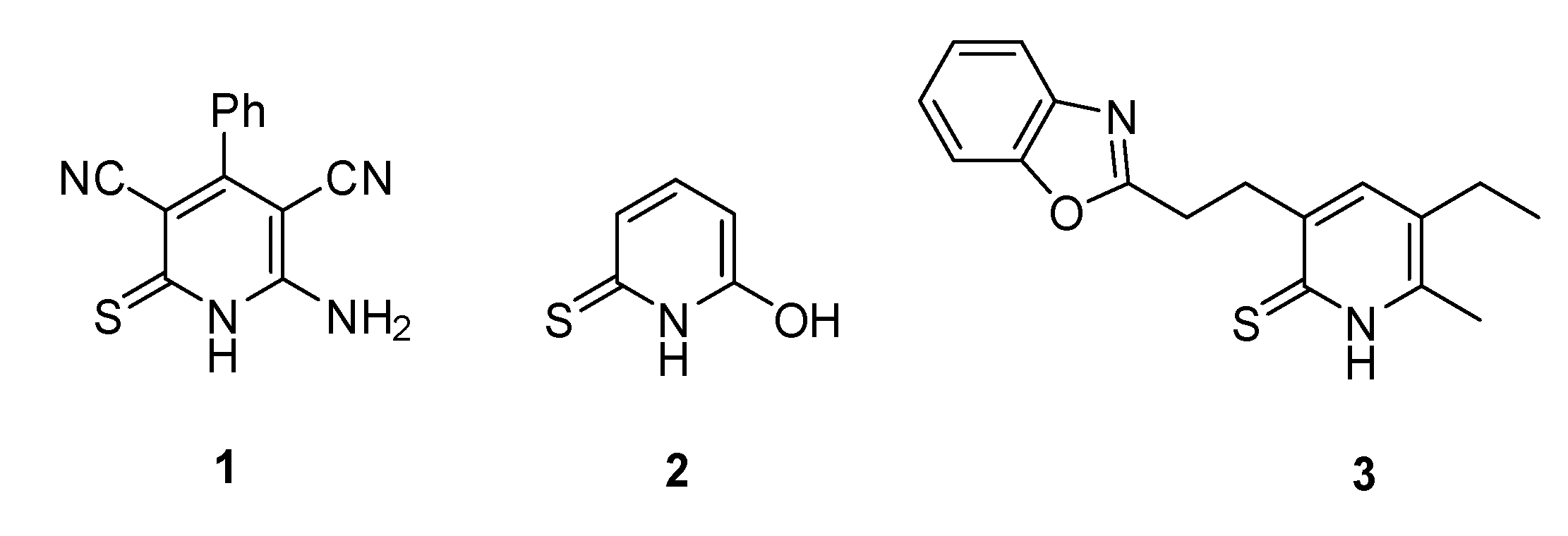
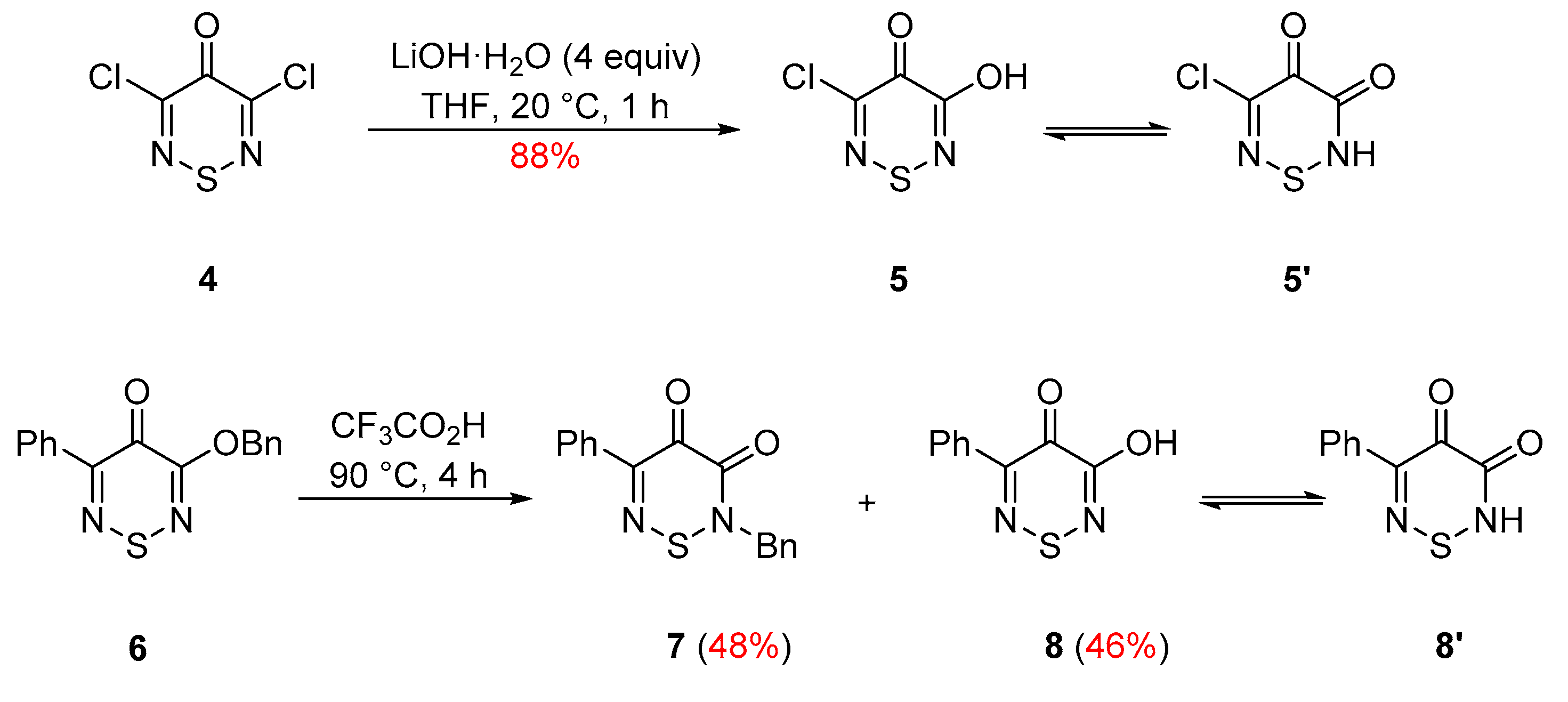
2. Results and Discussion
3. Materials and Methods
5,5′-Thiobis(3-methoxy-4H-1,2,6-thiadiazin-4-one) (10)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-3-chloro-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,097,594A, 27 June 1978. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-thio-3-chloro-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,100,281A, 27 June 1978. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. 3-Chloro-5-(optionally substituted heterocycloxy)-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,143,138, 3 March 1979. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-3-chloro-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,201,780, 6 May 1980. [Google Scholar]
- Portnoy, R.C. Thiadiazinone Plant Disease Control Agents. U.S. Patent 4,497,807A, 5 February 1985. [Google Scholar]
- Gómez, T.; Macho, S.; Miguel, D.; Neo, A.G.; Rodríguez, T.; Torroba, T. Cyclopentathiadiazines, Cyclohepta- and Cyclopentadithiazoles: New Materials and a Rich Heterocyclic Chemistry of Cyclic Enaminonitriles. Eur. J. Org. Chem. 2005, 5055–5066. [Google Scholar] [CrossRef]
- Macho, S.; Miguel, D.; Neo, A.G.; Rodríguez, T.; Torroba, T. Cyclopentathiadiazines, new heterocyclic materials from cyclic Enaminonitriles. Chem. Commun. 2005, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Lonchakov, A.V.; Rakitin, O.A.; Gritsan, N.P.; Zibarev, A.V. Breathing Some New Life into an Old Topic: Chalcogen-Nitrogen π-Heterocycles as Electron Acceptors. Molecules 2013, 18, 9850–9900. [Google Scholar] [CrossRef] [PubMed]
- Cava, M.P.; Lakshmikantham, M.V.; Hoffmann, R.; Williams, R.M.R.B. Woodward’s unfinished symphony: Designing organic superconductors (1975–79). Tetrahedron 2011, 67, 6771–6797. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W.; White, A.J.P.; Williams, D.J. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. Chem. Commun. 2000, 303–304. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. J. Chem. Soc. Perkin Trans. 1 2000, 1089–1094. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Cyclisation chemistry of 4H-1,2,6-thiadiazines. J. Chem. Soc. Perkin Trans. 1 2000, 2601–2607. [Google Scholar] [CrossRef]
- Theodorou, E.; Ioannidou, H.A.; Ioannou, T.A.; Kalogirou, A.S.; Constantinides, C.P.; Manoli, M.; Koutentis, P.A.; Hayes, S.C. Spectroscopic characterization of C-4 substituted 3,5-dichloro-4H-1,2,6-thiadiazines. RSC Adv. 2015, 51, 18471–18481. [Google Scholar] [CrossRef]
- Asquith, C.R.M.; Godoi, P.H.; Couñago, R.M.; Laitinen, T.; Scott, J.W.; Langendorf, C.G.; Oakhill, J.S.; Drewry, D.H.; Zuercher, W.J.; Koutentis, P.A.; et al. 1,2,6-Thiadiazinones as Novel Narrow Spectrum Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CaMKK2) Inhibitors. Molecules 2018, 23, 1221. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Koutentis, P.A. The chemistry of non-S-oxidised 4H-1,2,6-thiadiazines. Targets Heterocycl. Syst. 2018, 22, 82–118. [Google Scholar] [CrossRef]
- Samadi, A.; Marco-Contelles, J.; Soriano, E.; Álvarez-Pérez, M.; Chioua, M.; Romero, A.; González-Lafuente, L.; Gandía, L.; Roda, J.M.; López, M.G.; et al. Multipotent drugs with cholinergic and neuroprotective properties for the treatment of Alzheimer and neuronal vascular diseases. I. Synthesis, biological assessment, and molecular modeling of simple and readily available 2-aminopyridine-, and 2-chloropyridine-3,5-dicarbonitriles. Bioorg. Med. Chem. 2010, 18, 5861–5872. [Google Scholar] [CrossRef] [PubMed]
- Adamek, R.N.; Credille, C.V.; Dick, B.L.; Cohen, S.M. Isosteres of hydroxypyridinethione as drug-like pharmacophores for metalloenzyme inhibition. J. Biol. Inorg. Chem. 2018, 23, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Smith, A.M.; Rooney, C.S.; Fisher, T.E.; Wai, J.S.; Thomas, C.M.; Bamberger, D.L.; Barnes, J.L.; Williams, T.M.; Jones, J.H.; et al. Synthesis and Evaluation of 2-Pyridinone Derivatives as HIV-1-Specific Reverse Transcriptase Inhibitors. 3-[2-(Benzoxazol-2-yl)ethyl]-5-ethyl-6-methylpyridin-2(lfl)-one and Analogues. J. Med. Chem. 1993, 36, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, H.A.; Kizas, C.; Koutentis, P.A. Selective Stille Coupling Reactions of 3-Chloro-5-halo(pseudohalo)-4H-1,2,6-thiadiazin-4-ones. Org. Lett. 2011, 13, 5886–5889. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, H.A.; Koutentis, P.A. Synthesis of asymmetric 3,5-diaryl-4H-1,2,6-thiadiazin-4-ones via Suzuki–Miyaura and Stille coupling reactions. Tetrahedron 2012, 68, 7380–7385. [Google Scholar] [CrossRef]
- Geevers, J.; Trompen, W.P. Synthesis and reactions of 3,5-dichloro-4H-1,2,6-thiadiazin-4-one. Recl. Trav. Chim. Pays-Bas 1974, 93, 270–272. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, A.S.; Koutentis, P.A. 5,5′-Thiobis(3-methoxy-4H-1,2,6-thiadiazin-4-one). Molbank 2019, 2019, M1064. https://doi.org/10.3390/M1064
Kalogirou AS, Koutentis PA. 5,5′-Thiobis(3-methoxy-4H-1,2,6-thiadiazin-4-one). Molbank. 2019; 2019(2):M1064. https://doi.org/10.3390/M1064
Chicago/Turabian StyleKalogirou, Andreas S., and Panayiotis A. Koutentis. 2019. "5,5′-Thiobis(3-methoxy-4H-1,2,6-thiadiazin-4-one)" Molbank 2019, no. 2: M1064. https://doi.org/10.3390/M1064
APA StyleKalogirou, A. S., & Koutentis, P. A. (2019). 5,5′-Thiobis(3-methoxy-4H-1,2,6-thiadiazin-4-one). Molbank, 2019(2), M1064. https://doi.org/10.3390/M1064





