Abstract
Stille coupling of 3,5-bis(5-bromothien-2-yl)-4H-1,2,6-thiadiazin-4-one (10) with 2-(tri-n-butylstannyl)thiazole and Pd(Ph3P)2Cl2 in PhMe, at ca. 110 °C, for 2 h, gave 3,5-bis[5-(thiazol-2-yl)thien-2-yl]-4H-1,2,6-thiadiazin-4-one (9) in 81% yield. The latter is evaluated for its electronic properties.
1. Introduction
Heteroaromatic multicyclic molecules have uses in medicinal chemistry and materials science. Thiophene-containing oligomers, in particular, have been investigated in medicinal chemistry as potential anti-Alzheimer [1] and anti-cancer agents [2], while in materials science they are used as donors for organic photovoltaics (OPVs) [3,4]. Polycyclic small molecules containing rare heterocycles such as non-oxidized 4H-1,2,6-thiadiazines are uncommon but have potential uses as the electron poor thiadiazine unit can modulate the electronic and structural properties.
4H-1,2,6-Thiadiazines have interesting applications: selected 5-substituted 3-chloro-4H-1,2,6-thiadiazines displayed plant antifungal activity [5,6,7,8,9], while others displayed liquid crystalline properties or behaved as near-infrared dyes [10,11]. Furthermore, selected 4H-1,2,6-thiadiazines have been proposed as radical anion precursors for molecule-based magnetic and conducting materials [12], while π-conjugated polymers of 1,2,6-thiadiazines have been proposed as potentially stable alternatives to the superconductor poly(sulfur nitride) (SN)x by both Woodward [13] and Rees [14,15,16]. 4H-1,2,6-Thiadiazines were also characterized by resonance Raman (RR), absorption (UV-vis) and photoluminescence (PL) spectroscopies to better understand their optical properties [17]. Recently, we investigated the 1,2,6-thiadiazinone derivatives as narrow spectrum calcium/calmodulin-dependent protein kinase kinase 2 (CaMKK2) inhibitors as mimics of 2,4-dianilinopyrimidines [18], demonstrating a new medicinal chemistry application of the system.
A series of polycyclic small molecules containing non-S-oxidized 4H-1,2,6-thiadiazin-4-ones 2–6 were also prepared starting from 3,5-dichloro-4H-1,2,6-thiadiazin-4-one (1) and investigated as electron donors in solution-processed bulk heterojunction (BHJ) OPVs (Figure 1) [19]. Compounds 2–6, along with tetrathiophene 7 [20] and bithiadiazine 8 [21] (Figure 1) are the only known analogues containing a 1,2,6-thiadiazine ring. These compounds have all been prepared via Pd-catalyzed C-C bond coupling reactions and all contain multiple thiophenes resembling previously reported oligothiophenes [1,2,3] with a plethora of applications. Interestingly, the small molecule donors 2–6 synthesized in combination with PC70BM were used in BHJ solar cells with power conversion efficiencies (PCE) of ~3%, which is a good starting point for investigation in thiadiazine-containing OPVs. This prompted us to further investigate the synthesis of pentacyclic thiadiazines by introducing thiazole rings, which are isosters to thiophenes.
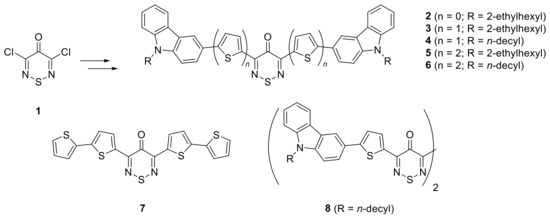
Figure 1.
Known 1,2,6-thiadiazine containing polycyclic small molecules.
2. Results and Discussion
Recent work by Leclerc et al. revealed that thiazoles are a useful substitute to thiophenes in OPVs as the more electron-deficient nature of the thiazole ring lowered the the lowest unoccupied molecular orbital (LUMO) values of donor oligomers thereby increasing the Voc and PCE of the BHJ devices [22]. Therefore, to evaluate the effect of introducing thiazoles in our thiadiazine-containing small molecules we pursued the synthesis of pentacyclic compound 9 (Scheme 1), an isoster of the tetrathiophene 7. The electrochemical properties of the latter were investigated in an electrochemical study of selected 1,2,6-thiadiazines [23], that would make it easy to compare the electrochemical properties of the two compounds.
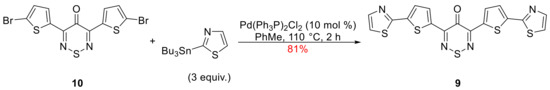
Scheme 1.
Synthesis of 3,5-bis [5-(thiazol-2-yl)thien-2-yl]-4H-1,2,6-thiadiazin-4-one (9).
Moreover, thiazyl 9 has some resemblance to pyridine-oxazole oligomers investigated as DNA G-quadruplex (G4) binders with potential uses as anticancer agents [24]. The similarity to our system is that 3,5-diarylthiadiazinones have the same geometry as the 2,6-diarylpyridines used in this study as both can adopt angular geometry. Furthermore, since thiazole is a non-classical bioisostere to oxazole, we can expect the two systems to show similar diamolecular interactions with the DNA target. The medicinal chemistry aspect of this work has not yet been investigated.
The Pd coupling chemistry (Stille) of 3,5-bis(5-bromothien-2-yl)-4H-1,2,6-thiadiazin-4-one (10) has been investigated as it was previously used to prepare the tetrathiopene 7 [20] as well as analogues 3–6 [19] and a small library of copolymers [25] investigated in OPVs. The Stille coupling reaction of bromothiophene 10 with 2-(tri-n-butylstannyl)thiazole proceeded smoothly to give the expected thiazyl 9 in 81% yield (Scheme 1).
The ultraviolet-visible (UV-vis) absorption spectrum of the pentacyclic thiazyl 9 was measured in solution (CH2Cl2) and gave a lowest energy absorption peak at λmax at 470 nm with an onset value of 616 nm corresponding to an optical band gap (EgOpt) of 2.34 eV (Table 1), which resembles the value of tetrathiophene 7 of 2.30 eV. The small band gap of the pentacyclic thiazyl as well as the broad absorption between 300–550 nm (see SI), which overlaps with the absorption regime of PC70BM (350–500 nm) [26], shows that molecule 9 was suitable as a donor for bulk heterojunction solar cells.

Table 1.
CV and UV-vis data of 3,5-bis[5-(thiazol-2-yl)thien-2-yl]-4H-1,2,6-thiadiazin-4-one (9) and 3,5-di[(2,2′-bithien)]-5-yl]-4H-1,2,6-thiadiazin-4-one (7). All the values correspond to peak onsets.
Thiazyl 9 was also analysed using cyclic voltammetry (CV) that revealed two reductions, one reversible and one quasi-reversible, while no oxidations were observed (see SI). The molecule showed an electrochemical LUMO value of −3.66 eV, while a highest occupied molecular orbital (HOMO) value was calculated to be −5.93 eV based on the optical band gap (EgOpt) (Table 1). Interestingly, the LUMO energy was favorably lower than that of tetrathiophene 7, illustrating that incorporation of the electron-deficient thiazole ring shifts the reduction to more negative potentials as the compound becomes more difficult to reduce. This shift represents a step in the right direction as the ideal LUMO of donors for OPVs is required to be as low as −4.0 eV to achieve better PCEs in organic photovoltaics (OPVs) that use PCBM as the acceptor [27].
This study shows that thiazole-containing thiadiazines can be readily synthesized and are worthy of further investigation as components of OPVs and for potential medicinal chemistry applications.
3. Materials and Methods
The reaction mixture was monitored by TLC using commercial glass backed thin layer chromatography (TLC) plates (Merck Kieselgel 60 F254, Darmstadt, Germany). The plates were observed under UV light at 254 and 365 nm. Toluene was distilled over CaH2 before use. The melting point was determined using a PolyTherm-A, Wagner & Munz, Kofler–Hotstage Microscope apparatus (Wagner & Munz, Munich, Germany). The solvent used for recrystallization is indicated after the melting point. The UV-vis spectrum was obtained using a Perkin–Elmer Lambda-25 UV-vis spectrophotometer (Perkin–Elmer, Waltham, MA, USA) and inflections are identified by the abbreviation “inf”. The IR spectrum was recorded on a Shimadzu FTIR-NIR Prestige-21 spectrometer (Shimadzu, Kyoto, Japan) with Pike Miracle Ge ATR accessory (Pike Miracle, Madison, WI, USA) and strong, medium and weak peaks are represented by s, m and w, respectively. 1H and 13C-NMR spectra were recorded on a Bruker Avance 500 machine (at 500 and 125 MHz, respectively, (Bruker, Billerica, MA, USA). Deuterated solvents were used for homonuclear lock and the signals are referenced to the deuterated solvent peaks. Attached proton test (APT) NMR studies identified carbon multiplicities, which are indicated by (s), (d), (t) and (q) notations. The MALDI-TOF mass spectrum (+ve mode) was recorded on a Bruker Autoflex III Smartbeam instrument (Bruker). The elemental analysis was run by the London Metropolitan University Elemental Analysis Service. 3,5-Bis(5-bromothien-2-yl)-4H-1,2,6-thiadiazin-4-one (10) was prepared according to the literature procedure [20].
3,5-Bis[5-(thiazol-2-yl)thien-2-yl]-4H-1,2,6-thiadiazin-4-one (9)
To a stirred mixture of 3,5-bis(5-bromothien-2-yl)-4H-1,2,6-thiadiazin-4-one (10) (43.6 mg, 0.100 mmol) in PhMe (1 mL) at ca. 20 °C was added 2-(tri-n-butylstannyl)thiazole (112 mg, 0.300 mmol) and Pd(Ph3P)2Cl2 (7 mg, 0.01 mmol). The solution was then deaerated by bubbling Ar gas into the reaction mixture for 10 min and then the mixture was heated at reflux under Ar, until no starting material remained (TLC, 2 h). On cooling to ca. 20 °C, t-BuOMe (10 mL) was added and the mixture was washed with saturated KF(aq), dried over Na2SO4, then adsorbed onto silica and chromatographed (t-BuOMe) to give the title compound 9 (35.9 mg, 81%) as red needles, mp 235–237 °C (from benzene); Rf 0.42 (t-BuOMe); (found: C, 45.90; H, 1.72; N, 12.62. C17H8N4OS5 requires C, 45.93; H, 1.81; N, 12.60%); λmax(CH2Cl2)/nm 300 (log ε 4.21), 354 (4.17), 470 (4.69), 489 inf (4.65); vmax/cm−1 3069w (C-H), 1653w, 1624m, 1558m, 1539m, 1506m, 1485m, 1477m, 1445s, 1425m, 1404m, 1368m, 1329m, 1273m, 1259m, 1242s, 1231s, 1148m, 1051s, 909m, 868s, 810s, 781m, 737m; δH (500 MHz; CDCl3) 8.26 (2H, d, J 4.2, Ar CH), 7.86 (2H, d, J 3.3, Ar CH), 7.61 (2H, d, J 4.1, Ar CH), 7.36 (2H, d, J 3.2, Ar CH); δC(125 MHz; CDCl3) 161.4 (s), 161.1 (s), 153.6 (s), 144.0 (d), 143.8 (s), 137.5 (s), 132.9 (d), 126.8 (d), 119.4 (d); m/z (MALDI-TOF) 445 (M+ + 1, 41%), 444 (M+, 20), 225 (63), 224 (100), 193 (61).
Supplementary Materials
The following are available online, mol file, Cyclic Voltammogram, UV-vis spectrum, 1H and 13C-NMR spectra.
Author Contributions
P.A. Koutentis conceived the experiments; A.S. Kalogirou designed and performed the experiments, analyzed the data and wrote the paper.
Funding
Cyprus Research Promotion Foundation (Grants: ΣΤΡΑΤΗΙΙ/0308/06, NEKYP/0308/02 ΥΓΕΙΑ/0506/19 and ΕΝΙΣΧ/0308/83).
Acknowledgments
The authors thank the following organizations and companies in Cyprus for generous donations of chemicals and glassware: the State General Laboratory, the Agricultural Research Institute, the Ministry of Agriculture, MedoChemie Ltd, Medisell Ltd and Biotronics Ltd. Furthermore, we thank the A. G. Leventis Foundation for helping to establish the NMR facility at the University of Cyprus.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Civitelli, L.; Sandin, L.; Nelson, E.; Khattak, S.E.; Brorsson, X.A.; Kågedal, K. The Luminescent Oligothiophene p-FTAA Converts Toxic Aβ1–42 Species into Nontoxic Amyloid Fibers with Altered Properties. J. Biol. Chem. 2016, 291, 9233–9243. [Google Scholar] [CrossRef]
- Yang, G.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. A Multifunctional Cationic Pentathiophene: Synthesis, Organelle-Selective Imaging, and Anticancer Activity. Adv. Funct. Mater. 2012, 22, 736–743. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, X.; Long, G. High Performance Photovoltaic Applications Using Solution-Processed Small Molecules. Acc. Chem. Res. 2013, 46, 2645–2655. [Google Scholar] [CrossRef]
- Tang, W.; Hai, J.; Dai, Y.; Huang, Z.; Lu, B.; Yuan, F.; Tang, J.; Zhang, F. Recent development of conjugated oligomers for high-efficiency bulk-heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1963–1979. [Google Scholar] [CrossRef]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-3-chloro-4H-1,2,6-thiadiazin-4-one Antifungal Agents. U.S. Patent 4,097,594, 27 June 1978. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-thio-3-chloro-4H-1,2,6-thiadiazin-4-one antifungal agents. U.S. Patent 4,100,281, 11 July 1978. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. 3-Chloro-5-(optionally substituted heterocycloxy)-4H-1,2,6-thiadiazin-4-one Antifungal Agents. U.S. Patent 4,143,138, 6 March 1979. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-3-chloro-4H-1,2,6-thiadiazin-4-one Antifungal Agents. U.S. Patent 4,201,780, 6 May 1980. [Google Scholar]
- Portnoy, R.C. Thiadiazinone Plant Disease Control Agents. U.S. Patent 4,497,807, 5 February 1985. [Google Scholar]
- Gómez, T.; Macho, S.; Miguel, D.; Neo, A.G.; Rodríguez, T.; Torroba, T. Cyclopentathiadiazines, Cyclohepta- and Cyclopentadithiazoles: New Materials and a Rich Heterocyclic Chemistry of Cyclic Enaminonitriles. Eur. J. Org. Chem. 2005, 2005, 5055–5066. [Google Scholar] [CrossRef]
- Macho, S.; Miguel, D.; Neo, A.G.; Rodríguez, T.; Torroba, T. Cyclopentathiadiazines, new heterocyclic materials from cyclic Enaminonitriles. Chem. Commun. 2005, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Lonchakov, A.V.; Rakitin, O.A.; Gritsan, N.P.; Zibarev, A.V. Breathing Some New Life into an Old Topic: Chalcogen-Nitrogen π-Heterocycles as Electron Acceptors. Molecules 2013, 18, 9850–9900. [Google Scholar] [CrossRef]
- Cava, M.P.; Lakshmikantham, M.V.; Hoffmann, R.; Williams, R.M. R. B. Woodward’s unfinished symphony: Designing organic superconductors (1975–79). Tetrahedron 2011, 67, 6771–6797. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W.; White, A.J.P.; Williams, D.J. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. Chem. Commun. 2000, 303. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. J. Chem. Soc. Perkin Trans. 1 2000, 1089–1094. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Cyclisation chemistry of 4H-1,2,6-thiadiazines. J. Chem. Soc. Perkin Trans. 1 2000, 2601–2607. [Google Scholar] [CrossRef]
- Theodorou, E.; Ioannidou, H.A.; Ioannou, T.A.; Kalogirou, A.S.; Constantinides, C.P.; Manoli, M.; Koutentis, P.A.; Hayes, S.C. Spectroscopic characterization of C-4 substituted 3,5-dichloro-4H-1,2,6-thiadiazines. RSC Adv. 2015, 51, 18471–18481. [Google Scholar] [CrossRef]
- Asquith, C.R.M.; Godoi, P.H.; Couñago, R.M.; Laitinen, T.; Scott, J.W.; Langendorf, C.G.; Oakhill, J.S.; Drewry, D.H.; Zuercher, W.J.; Koutentis, P.A.; et al. 1,2,6-Thiadiazinones as Novel Narrow Spectrum Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CaMKK2) Inhibitors. Molecules 2018, 23, 1221. [Google Scholar] [CrossRef] [PubMed]
- Hermerschmidt, F.; Kalogirou, A.S.; Min, J.; Zissimou, G.A.; Tuladhar, S.M.; Ameri, T.; Faber, H.; Itskos, G.; Choulis, S.A.; Anthopoulos, T.D.; et al. 4H-1,2,6-Thiadiazin-4-one-containing small molecule donors and additive effects on their performance in solution-processed organic solar cells. J. Mater. Chem. C 2015, 3, 2358–2365. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Kizas, C.; Koutentis, P.A. Palladium Catalyzed C-C Coupling Reactions of 3,5-Dichloro-4H-1,2,6-thiadiazin-4-one. Org. Lett. 2011, 13, 3466–3469. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Koutentis, P.A. 5,5′-Bis[5-(9-decyl-9H-carbazol-3-yl)thien-2-yl]-4H,4′H-[3,3′-bi(1,2,6-thia-diazine)]-4,4′-dione. Molbank 2018, 2018, M987. [Google Scholar] [CrossRef]
- Bulut, I.; Chavez, P.; Mirloup, A.; Huaulme, Q.; Hebraud, A.; Heinrich, B.; Fall, S.; Mery, S.; Ziessel, R.; Heiser, T.; et al. Thiazole-based scaffolding for high performance solar cells. J. Mater. Chem. C 2016, 4, 4296–4303. [Google Scholar] [CrossRef]
- Economopoulos, S.P.; Koutentis, P.A.; Ioannidou, H.A.; Choulis, S.A. Identifying potential candidates for donor–acceptor copolymers on a series of 4H-1,2,6-thiadiazines: An electrochemical approach. Electrochim. Acta 2013, 107, 448–453. [Google Scholar] [CrossRef]
- Verga, D.; NGuyen, C.-H.; Dakir, M.-D.; Coll, J.-L.; Teulade-Fichou, M.-P.; Molla, A. Polyheteroaryl Oxazole/Pyridine-Based Compounds Selected in Vitro as G-Quadruplex Ligands Inhibit Rock Kinase and Exhibit Antiproliferative Activity. J. Med. Chem. 2018, 61, 10502–10518. [Google Scholar] [CrossRef]
- Chochos, C.L.; Kalogirou, A.S.; Ye, T.; Tatsi, E.; Katsouras, A.; Zissimou, G.A.; Gregoriou, V.G.; Avgeropoulos, A.; Koutentis, P.A. 4H-1,2,6-Thiadiazine-containing donor–acceptor conjugated polymers: Synthesis, optoelectronic characterization and their use in organic solar cells. J. Mater. Chem. C 2018. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, K.; Coates, N.E.; Moses, D.; Nguyen, T.-Q.; Dante, M.; Heeger, A.J. Efficient tandem polymer solar cells fabricated by all-solution processing. Science 2007, 317, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).