N-Propargylation of Indolo-Triterpenoids and Their Application in Mannich Reaction
Abstract
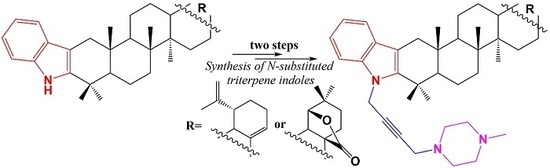
:1. Introduction
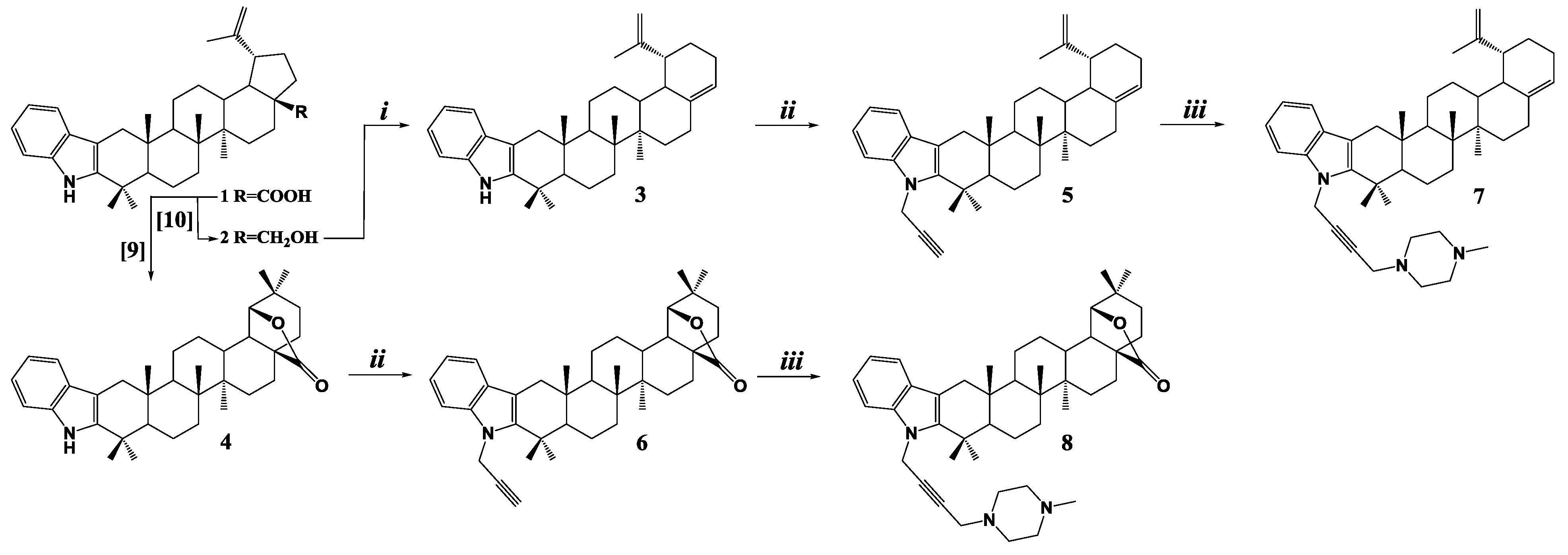
2. Results
3. Materials and Methods
3.1. [3,2b]Indolo-lup-20(29),17(28)-dien (3)
3.2. [3,2b]Indolo-N-prop-2-yn-1-yl-lup-20(29),17(28)-dien (5)
3.3. [3,2b]Indolo-N-prop-2-yn-1-yl-28-oxo-allobetulon (6)
3.4. [3,2b]Indolo-N-(4-(4-methylpiperazin-1-yl)but-2-yn-1-yl)- lup-20(29),17(28)-dien (7)
3.5. [3,2b]Indolo-N-(4-(4-methylpiperazin-1-yl)but-2-yn-1-yl)-28-oxo-allobetulon (8)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Gonçalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.L.C.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Recent developments in the functionalization of betulinic acid and its natural analogues: a route to new bioactive compounds. Molecules 2019, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Kvasnica, M.; Urban, M.; Dickinson, N.J.; Sarek, J. Pentacyclic triterpenoids with nitrogen- and sulfur-containing heterocycles: synthesis and medicinal significance. Nat. Prod. Rep. 2015, 32, 1303–1330. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, H.C.; Verma, A.K.; Choi, E.H. Biomedical importance of indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef]
- Qiu, W.W.; Shen, Q.; Yang, F.; Wang, B.; Zou, H.; Li, J.Y.; Li, J.; Tang, J. Synthesis and biological evaluation of heterocyclic ring-substituted maslinic acid derivatives as novel inhibitors of protein tyrosine phosphatase 1B. Bioorg. Med. Chem. Lett. 2009, 19, 6618–6622. [Google Scholar] [CrossRef]
- Gu, W.; Hao, Y.; Zhang, G.; Wang, S.F.; Miao, T.T.; Zhang, K.P. Synthesis, in vitro antimicrobial and cytotoxic activities of new carbazole derivatives of ursolic acid. Bioorg. Med. Chem. Lett. 2015, 25, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Rani, N.; Aggarwal, P.; Sanna, V.K.; Singh, A.T.; Jaggi, M.; Joshi, N.; Sharma, P.K.; Irchhaiya, R.; Burman, A.C. Synthesis and cytotoxic activity of heterocyclic ring-substituted betulinic acid derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 5058–5062. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.F.; Smirnova, I.E.; Giniyatullina, G.V.; Medvedeva, N.I.; Yamansarov, E.Y.; Kazakov, D.V.; Kazakova, O.B.; Linh, P.T.; Viet, do Q.; Huong, D.T. Inhibition of alpha-glucosidase by synthetic derivatives of lupane, oleanane, ursane and dammarane triterpenoids. Nat. Prod. Commun. 2016, 11, 33–35. [Google Scholar] [PubMed]
- Khusnutdinova, E.F.; Smirnova, I.E.; Kazakova, O.B.; Petrova, A.V.; Nguyen, T.T.H. Synthesis and evaluation of 2,3-indolotriterpenoids as new α-glucosidase inhibitors. Med. Chem. Res. 2017, 26, 2737–2742. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Nguyen, T.T.H.; Le, T.T.A.; Nguyen, T.T.; Ba, T.C.; Babkov, D.A.; Kazakova, O.B. Structural modifications of 2,3-indolobetulinic acid: Design and synthesis of highly potent α-glucosidase inhibitors. Bioorg. Chem. 2019, 88. in press. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Apryshko, G.N.; Kukovinets, O.S.; Kazakova, O.B. Synthesis and cytotoxicity of indole derivatives of betulin, erythrodiol, and uvaol. Russ. J. Bioorg. Chem. 2018, 44, 322–329. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Kazakova, O.B.; Lobov, A.N.; Kukovinets, O.S.; Suponitsky, K.Y.; Meyers, C.B.; Prichard, M.N. Synthesis of A-ring quinolones, nine-membered oxolactams and spiroindoles by oxidative transformations of 2,3-indolotriterpenoids. Org. Biomol. Chem. 2019, 17, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Hua, D.W.; Miao, T.T.; Wang, S.F.; Jin, X.Y.; Gu, W. Synthesis, crystal structure and antitumor activity of a new indolequinone derivative of ursolic acid. Chin. J. Struct. Chem. 2016, 35, 1167–1173. [Google Scholar]
- Pokorny, J.; Borkova, L.; Urban, M. Click reactions in chemistry of triterpenes–advance towards development of potential therapeutics. Curr. Med. Chem. 2018, 25, 636–658. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Deigner, H.P. The potential of click reactions for the synthesis of bioactive triterpenes. Bioorg. Med. Chem. Lett. 2019, 29, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.F.; Petrova, A.V.; Kukovinets, O.S.; Kazakova, O.B. Synthesis and cytotoxicity of 28-N-propargylaminoalkylated 2,3-indolotriterpenic acids. Nat. Prod. Commun. 2018, 13, 665–668. [Google Scholar] [CrossRef]
- Suman, P.; Patel, A.; Solano, L.; Jampan, G.; Gardner, Z.S.; Holt, G.M.; Jonnalagadda, S.C. Synthesis and cytotoxicity of baylis-hillman template derived betulinic acid-triazole conjugates. Tetrahedron 2017, 73, 4214–4226. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khusnutdinova, E.F.; Petrova, A.V.; Bashirova, G.M.; Kazakova, O.B. N-Propargylation of Indolo-Triterpenoids and Their Application in Mannich Reaction. Molbank 2019, 2019, M1065. https://doi.org/10.3390/M1065
Khusnutdinova EF, Petrova AV, Bashirova GM, Kazakova OB. N-Propargylation of Indolo-Triterpenoids and Their Application in Mannich Reaction. Molbank. 2019; 2019(2):M1065. https://doi.org/10.3390/M1065
Chicago/Turabian StyleKhusnutdinova, Elmira F., Anastasiya V. Petrova, Gulnaz M. Bashirova, and Oxana B. Kazakova. 2019. "N-Propargylation of Indolo-Triterpenoids and Their Application in Mannich Reaction" Molbank 2019, no. 2: M1065. https://doi.org/10.3390/M1065
APA StyleKhusnutdinova, E. F., Petrova, A. V., Bashirova, G. M., & Kazakova, O. B. (2019). N-Propargylation of Indolo-Triterpenoids and Their Application in Mannich Reaction. Molbank, 2019(2), M1065. https://doi.org/10.3390/M1065