(E)-3-(2,5-Dimethoxyphenyl)-1-{[4-(2,5-dimethoxy-phenyl)-6-((E)-2,5-dimethoxystyryl)-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl]}prop-2-en-1-one and (E)-3-(2,5-Dimethoxyphenyl)-1-{[4-(2,5-dimethoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl]}prop-2-en-1-one
Abstract
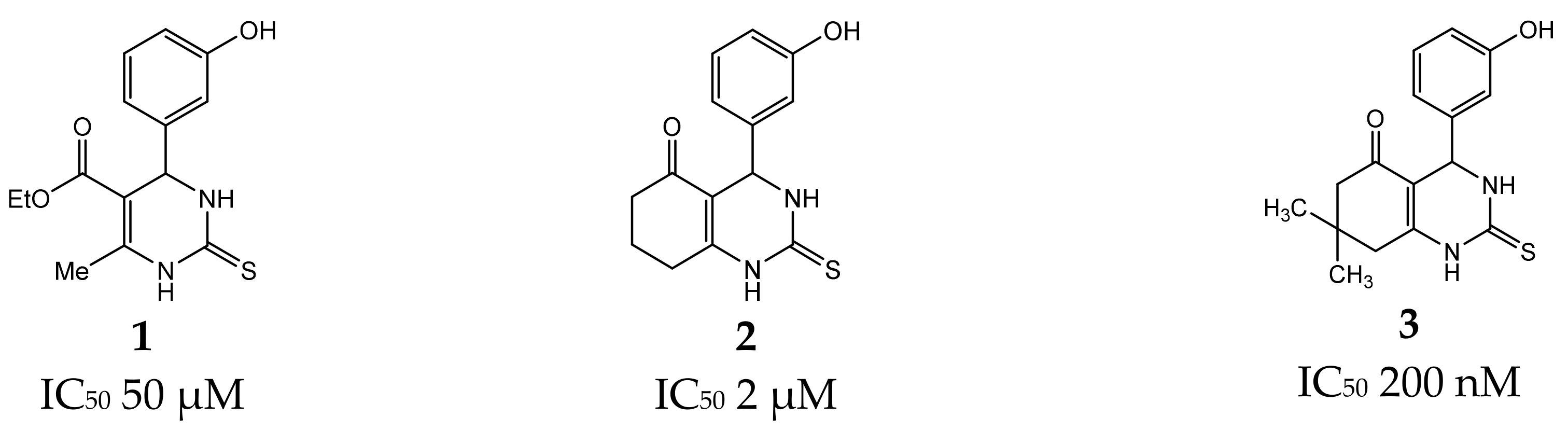
:1. Introduction
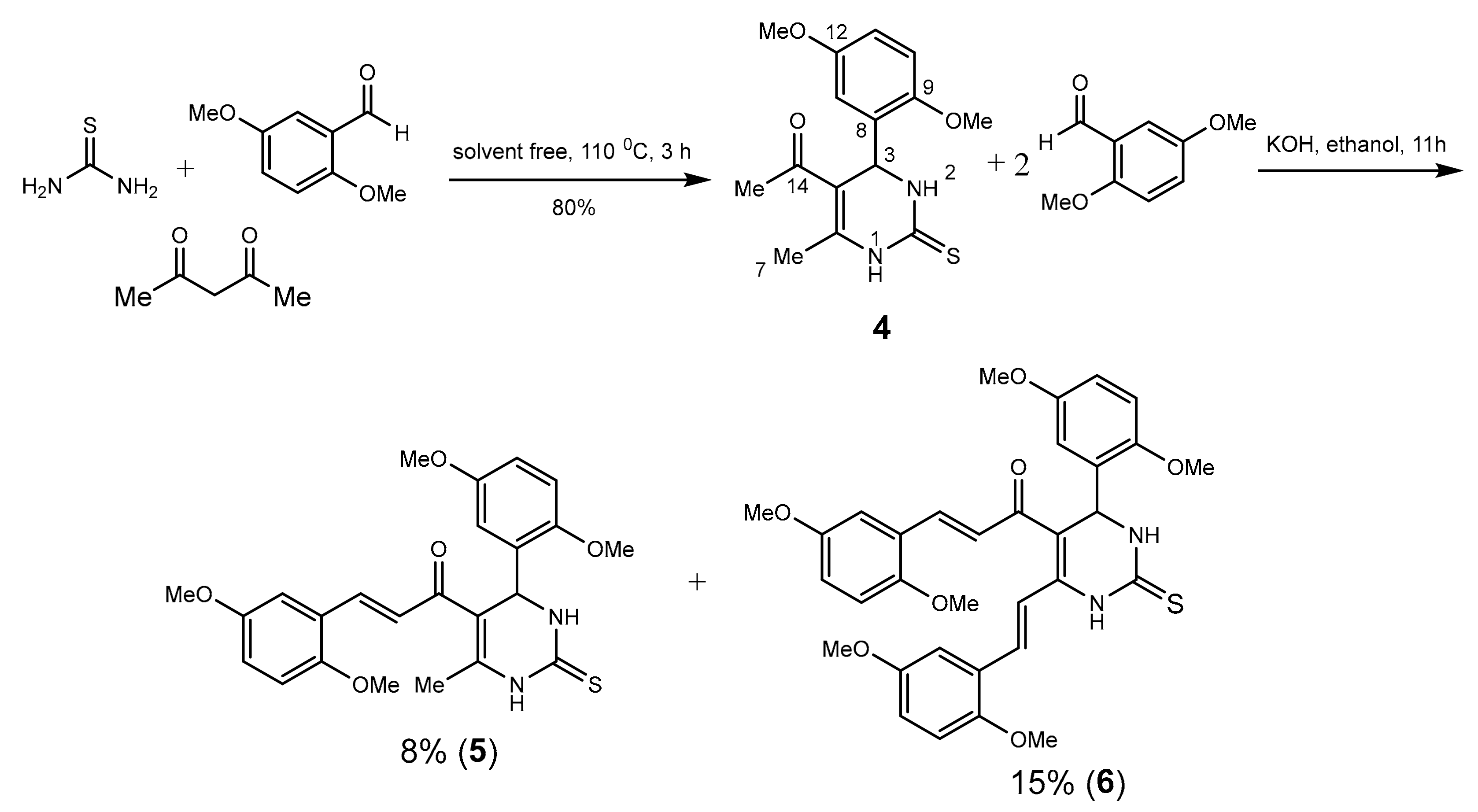
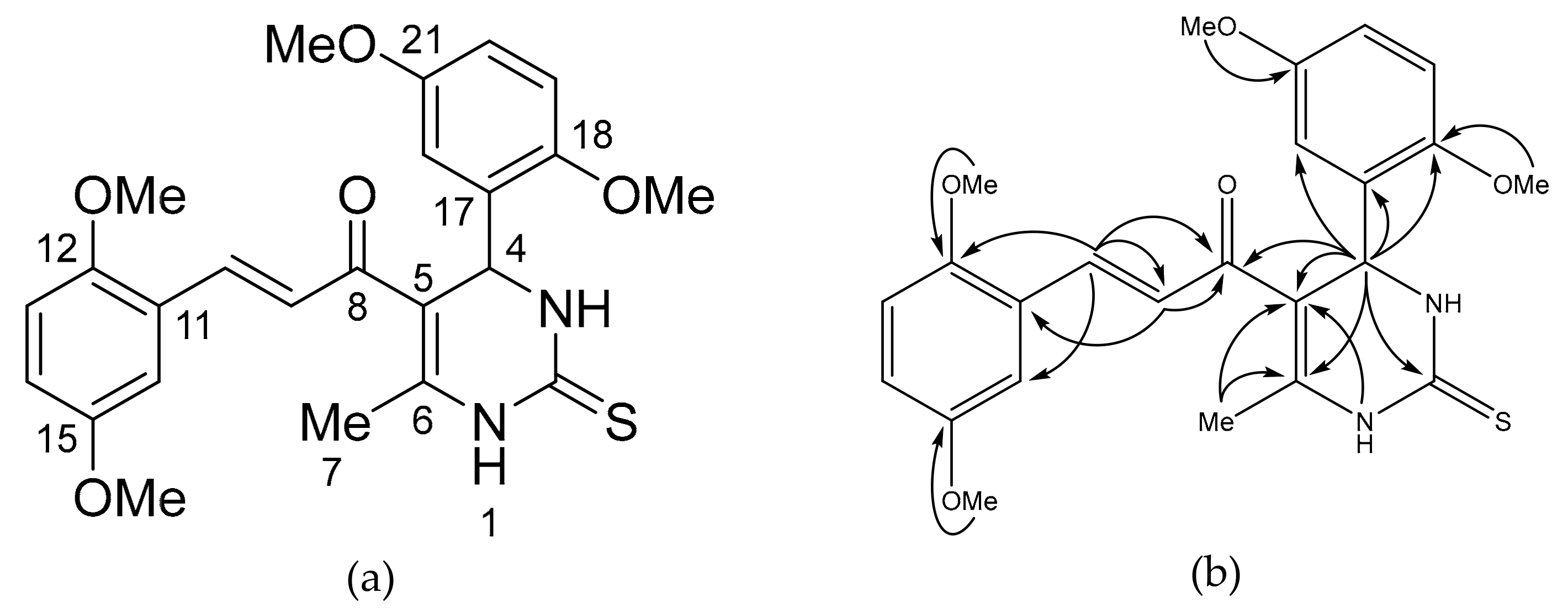
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of Compound 4
3.3. Synthesis of the Title Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sośnicki, J.G.; Struk, Ł.; Kurzawski, M.; Perużyńska, M.; Maciejewska, G.; dan Droździk, M. Regioselective Synthesis of Novel 4,5-diaryl functionalized 3,4-dihydropyrimidine-2(1H)-thiones Via a Non-Biginelli-Type Approach and Evaluation of Their in Vitro Anticancer Activity. Org. Biomol. Chem. 2014, 12, 3427–3440. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcelos, A.; Oliveira, P.S.; Ritter, M.; Freitag, R.A.; Romano, R.L.; Quina, F.H.; Pizzuti, L.; Pereira, C.M.P.; Stefanello, F.M.; Barschak, A.G. Antioxidant Capacity and Environmentally Friendly Synthesis of Dihydropyrimidin-(2H)-ones Promoted by Naturally Occurring Organic Acids. J. Biochem. Mol. Toxicol. 2012, 26, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Bhatewara, A.; Jetti, S.R.; Kadre, T.; Paliwal, P.; dan Jain, S. Microwave-assisted synthesis and biological evaluation of dihydropyrimidinone derivatives as anti-inflammatory, antibacterial, and antifungal agents. Int. J. Med. Chem. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, C.; Ok, T.; So, W.; Jo, M.; Seo, M.; Kim, Y.; Sohn, J.H.; Park, Y.; Ju, M.K.; et al. Discovery of 3,4-dihydropyrimidin-2(1H)-ones with Inhibitory Activity Against HIV-1 Replication. Bioorg. Med. Chem. Lett. 2012, 22, 2119–2124. [Google Scholar] [CrossRef] [PubMed]
- Nagarajaiah, H.; Mukhopadhyay, A.; dan Moorthy, J.N. Biginelli Reaction: An Overview. Tetrahedron Lett. 2016, 57, 5135–5149. [Google Scholar] [CrossRef]
- Lal, J.; Gupta, S.K.; Thavaselvam, D.; Agarwal, D.D. Design, synthesis, synergistic antimicrobial activity and cytotoxicity of 4-aryl substituted 3,4-dihydropyrimidinones of curcumin. Bioorg. Med. Chem. Lett. 2012, 22, 2872–2876. [Google Scholar] [CrossRef] [PubMed]
- Comas, H.; Buisson, D.A.; Najman, R.; Kozielski, F.; Rousseau, B.; Lopez, R. Synthesis of 5-acyl-3,4-dihydropyrimidine-2-thiones via solvent free, solvent-phase and solid-phase Biginelli procedures. Synlett 2009, 11, 1737–1740. [Google Scholar]
- Gartner, M.; Sunder-Plassmann, N.; Seiler, J.; Utz, M.; Vernos, I.; Surrey, T.; Giannis, A. Development and biological evaluation of potent and specific inhibitors of mitotic kinesin Eg5. ChemBioChem 2005, 6, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Kosolov, M.A.; Orlov, V.D.; Vashchenko, V.V.; Shishkina, S.V.; Shishkin, O.V. 5-Cynnamoyl- and 5-(ethoxycarbonyl)-6-styryl derivatives of 4-aryl-3,4-dihydropyrimidin-2(1H)-ones. Coll. Czech. Chem. Commun 2007, 72, 1219–1228. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 4th ed.; Brooks/Cole: Belmont, CA, USA, 2009; pp. 31–81. [Google Scholar]

| No. Atom | δH (Mult, J Hz) | δC (ppm) |
|---|---|---|
| 1 | 7.93 (s, 1H) | - |
| 2 | - | 174.7 |
| 3 | 7.46 (s, 1H) | - |
| 4 | 5.75 (d, J = 3.3 Hz, 1H) | 50.9 |
| 5 | - | 108.0 |
| 6 | - | 143.9 |
| 7 | 2.04 (s, 3H) | 19.3 |
| 8 | - | 129.0 |
| 9 | - | 154.0 |
| 9-OMe | 3.86 (s, 3H) | 56.3 |
| 10 | 6.84 (d, J = 9.0 Hz, 1H) | 114.4 |
| 11 | 6.79 (dd, J = 9.0, 3.0 Hz, 1H) | 113.2 |
| 12 | - | 150.6 |
| 12-OMe | 3.72 (s, 3H) | 55.8 |
| 13 | 6.59 (d, J = 3.0 Hz, 1H) | 111,5 |
| 14 | - | 195.8 |
| 15 | 2.40 (s, 3H) | 29.5 |
| No. Atom | δH (ppm), mult, J (Hz) | δC (ppm) | HMBC |
|---|---|---|---|
| 1 | 9.19 (s, 1H) | C-5 | |
| 2 | 176.64 | ||
| 3 | 8.30 (s, 1H) | ||
| 4 | 5.92 (d, J = 3.4 Hz, 1H) | 51.28 | C-2, C-5, C-6, C-8 C-17, C-18, C-22 |
| 5 | 110.86 | ||
| 6 | 143.63 | ||
| 7 | 2.46 (s, 3H) | 18.41 | C-5, C-6 |
| 8 | 189.05 | ||
| 9 | 7.18 (d, J = 15.8 Hz, 1H) | 126.76 | C-11 |
| 10 | 7.77 (d, J = 15.8 Hz, 1H) | 137.41 | C-8, C-9, C-12, C-16 |
| 11 | 125.26 | ||
| 12 | 153.89 | ||
| 12-OCH3 | 3.81 (s, 3H) | 56.39 | C-12 |
| 13 | 6.83–6.79 (m, 2H) | 113.54 | |
| 14 | 6.83–6.79 (m, 2H) | 117.76 | |
| 15 | 154.62 | ||
| 15-OCH3 | 3.77 (s, 3H) | 56.04 | C-15 |
| 16 | 7.13 (d, J = 2.6 Hz, 1H) | 113.97 | C-10, C-12, C-14 |
| 17 | 132.08 | ||
| 18 | 151.48 | ||
| 18-OCH3 | 3.84 (s, 3H) | 56.44 | C-18 |
| 19 | 6.97–6.94 (m, 2H) | 112.86 | |
| 20 | 6.97–6.94 (m, 2H) | 113.66 | |
| 21 | 154.80 | ||
| 21-OCH3 | 3.69 (s, 3H) | 55.79 | C-21 |
| 22 | 6.84 (d, J = 3.1 Hz, 1H) | 115.29 | C-18, C-20, C-21 |
| No. Atom | δH (ppm), mult, J (Hz) | δC (ppm) | HMBC |
|---|---|---|---|
| 1 | 10.36 (d, J = 1.4 Hz, 1H) | C-2, C-5, C-6, C-7 | |
| 2 | 175.10 | ||
| 3 | 9.41 (dd, J = 4.0, 1.4 Hz, 1H) | C-2, C-5 | |
| 4 | 5.59 (d, J = 4.0 Hz, 1H) | 49.91 | C-2, C-5, C-6, C-15, C-24, C-25 |
| 5 | 112.18 | ||
| 6 | 141.15 | ||
| 7 | 7.37 (d, J = 16.4 Hz, 1H) | 120.65 | C-6, C-8, C-9 |
| 8 | 7.43 (d, J = 16.4 Hz, 1H) | 131.76 | C-6, C-7, C-10 |
| 9 | 124.94 | ||
| 10 | 151.73 | ||
| 10-OCH3 | 3.69 (s, 3H) | 55.33 | C-10 |
| 11 | 6.95–6.93 (m, 3H) | 112.88 | |
| 12 | 6.89 (dd, J = 9.0, 3.0 Hz, 1H) | 116.07 | C-10 |
| 13 | 153.10 | ||
| 13-OCH3 | 3.64 (s, 3H) | 55.90 | C-13 |
| 14 | 7.11 (d, J = 3.0 Hz, 1H) | 112.31 | C-8, C-10, C-12 |
| 15 | 188.66 | ||
| 16 | 7.25 (d, J = 15.8 Hz, 1H) | 127.05 | C-15, C-18 |
| 17 | 7.59 (d, J = 15.8 Hz, 1H) | 135.04 | C-15, C-16, C-19 |
| 18 | 123.61 | ||
| 19 | 152.41 | ||
| 19-OCH3 | 3.68 (s, 3H) | 55.85 | C-19 |
| 20 | 6.95–6.93 (m, 3H) | 112.93 | |
| 21 | 6.95–6.93 (m, 3H) | 117.48 | |
| 22 | 153.21 | ||
| 22-OCH3 | 3.60 (s, 3H) | 55.91 | C-22 |
| 23 | 7.10 (d, J = 2.7 Hz, 1H) | 112.26 | C-17, C-21, C-25 |
| 24 | 130.79 | ||
| 25 | 150.33 | ||
| 25-OCH3 | 3.73 (s, 3H) | 55.29 | C-25 |
| 26 | 6.97 (d, J = 8.8 Hz, 1H) | 112.56 | |
| 27 | 6.85 (dd, J = 8.9, 3.1 Hz, 1H) | 112.32 | C-25, C-29 |
| 28 | 153.04 | ||
| 28-OCH3 | 3.66 (s, 3H) | 55.32 | C-28 |
| 29 | 6.70 (d, J = 3.0 Hz, 1H) | 114.06 | C-25, C-27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwito, H.; Kurnyawaty, N.; Susetyo, E.; Azizah, Y.; Haq, K.U.; Novi Kristanti, A.; Indriani, I. (E)-3-(2,5-Dimethoxyphenyl)-1-{[4-(2,5-dimethoxy-phenyl)-6-((E)-2,5-dimethoxystyryl)-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl]}prop-2-en-1-one and (E)-3-(2,5-Dimethoxyphenyl)-1-{[4-(2,5-dimethoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl]}prop-2-en-1-one. Molbank 2019, 2019, M1063. https://doi.org/10.3390/M1063
Suwito H, Kurnyawaty N, Susetyo E, Azizah Y, Haq KU, Novi Kristanti A, Indriani I. (E)-3-(2,5-Dimethoxyphenyl)-1-{[4-(2,5-dimethoxy-phenyl)-6-((E)-2,5-dimethoxystyryl)-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl]}prop-2-en-1-one and (E)-3-(2,5-Dimethoxyphenyl)-1-{[4-(2,5-dimethoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl]}prop-2-en-1-one. Molbank. 2019; 2019(2):M1063. https://doi.org/10.3390/M1063
Chicago/Turabian StyleSuwito, Hery, Noorma Kurnyawaty, Ellyca Susetyo, Yuzkiya Azizah, Kautsar Ul Haq, Alfinda Novi Kristanti, and Indriani Indriani. 2019. "(E)-3-(2,5-Dimethoxyphenyl)-1-{[4-(2,5-dimethoxy-phenyl)-6-((E)-2,5-dimethoxystyryl)-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl]}prop-2-en-1-one and (E)-3-(2,5-Dimethoxyphenyl)-1-{[4-(2,5-dimethoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl]}prop-2-en-1-one" Molbank 2019, no. 2: M1063. https://doi.org/10.3390/M1063
APA StyleSuwito, H., Kurnyawaty, N., Susetyo, E., Azizah, Y., Haq, K. U., Novi Kristanti, A., & Indriani, I. (2019). (E)-3-(2,5-Dimethoxyphenyl)-1-{[4-(2,5-dimethoxy-phenyl)-6-((E)-2,5-dimethoxystyryl)-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl]}prop-2-en-1-one and (E)-3-(2,5-Dimethoxyphenyl)-1-{[4-(2,5-dimethoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl]}prop-2-en-1-one. Molbank, 2019(2), M1063. https://doi.org/10.3390/M1063




