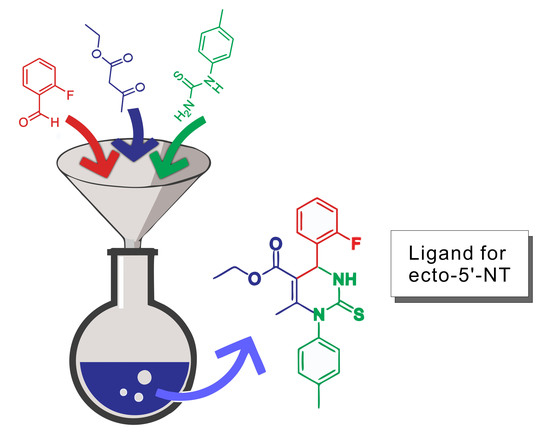
Ethyl 4-(2-fluorophenyl)-6-methyl-2-thioxo-1-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Analysis
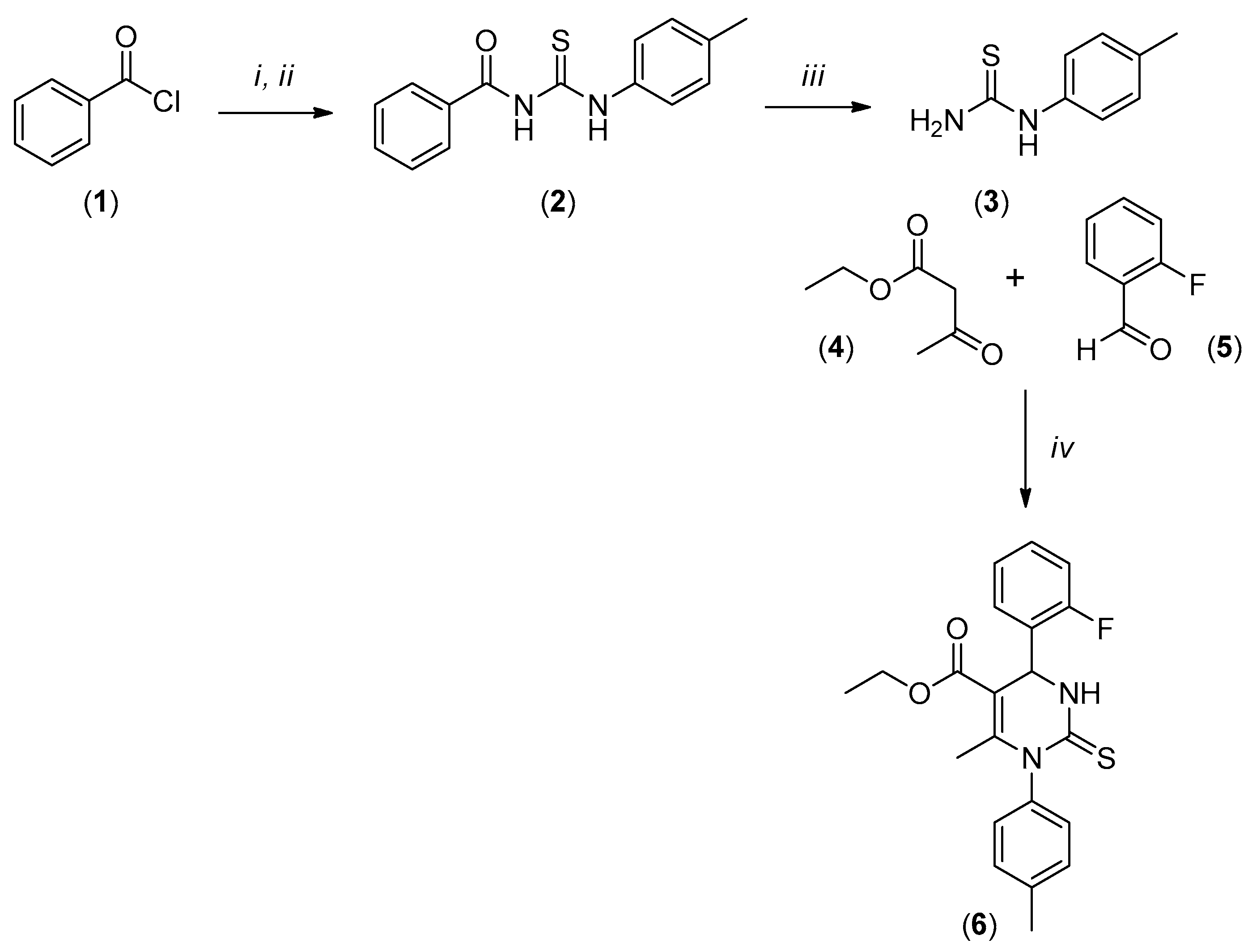
3.2. Synthesis of 1-(4-Methylphenyl)thiourea
3.3. Synthesis of Ethyl 4-(2-fluorophenyl)-6-methyl-2-thioxo-1-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate–LaSOM 282
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Recent synthetic and medicinal perspectives of dihydropyrimidinones: A review. Eur. J. Med. Chem. 2017, 132, 108–134. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.L.; Davi, L.; Rockenbach, L.; das Neves, G.M.; Kagami, L.P.; Canto, R.F.S.; Figueiró, F.; Battastini, A.M.O.; Eifler-Lima, V.L. Versatility of the Biginelli reaction: Synthesis of new biphenyl dihydropyrimidin-2-thiones using different ketones as building blocks. Tetrahedron Lett. 2018, 59, 2759–2762. [Google Scholar] [CrossRef]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.L.; Rockenbach, L.; das Neves, G.M.; Göethel, G.; Nascimento, F.; Kagami, L.P.; Figueiró, F.; de Azambuja, G.O.; de Fraga Dias, A.; Amaro, A. Effect of N-1 arylation of monastrol on kinesin Eg5 inhibition in glioma cell lines. MedChemComm 2018, 9, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Corbelini, P.F.; Figueiro, F.; das Neves, G.M.; Andrade, S.; Kawano, D.F.; Oliveira Battastini, A.M.; Eifler-Lima, V.L. Insights into Ecto-5′-Nucleotidase as a New Target for Cancer Therapy: A Medicinal Chemistry Study. Curr. Med. Chem. 2015, 22, 1776–1792. [Google Scholar] [CrossRef] [PubMed]
- Figueiro, F.; Mendes, F.B.; Corbelini, P.F.; Janarelli, F.; JANDREY, E.H.F.; Russowsky, D.; Eifler-Lima, V.L.; BATTASTINI, A.M.O. A Monastrol-derived Compound, LaSOM 63, Inhibits Ecto-5’Nucleotidase/CD73 Activity and Induces Apoptotic Cell Death of Glioma Cell Lines. Anticancer Res. 2014, 34, 1837–1842. [Google Scholar] [PubMed]
- Schüller, A.; Hähnke, V.; Schneider, G. SmiLib v2. 0: A Java-Based Tool for Rapid Combinatorial Library Enumeration. QSAR Comb. Sci. 2007, 26, 407–410. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, H.; Li, H. SHAFTS: A hybrid approach for 3D molecular similarity calculation. 1. Method and assessment of virtual screening. J. Chem. Inf. Model 2011, 51, 2372–2385. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, I.L.; Porto Kagami, L.; Machado das Neves, G.; Rockenbach, L.; Davi, L.; Soares, A.F.; Garcia, S.C.; Eifler-Lima, V.L. Ethyl 4-(2-fluorophenyl)-6-methyl-2-thioxo-1-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank 2018, 2018, M1029. https://doi.org/10.3390/M1029
Gonçalves IL, Porto Kagami L, Machado das Neves G, Rockenbach L, Davi L, Soares AF, Garcia SC, Eifler-Lima VL. Ethyl 4-(2-fluorophenyl)-6-methyl-2-thioxo-1-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank. 2018; 2018(4):M1029. https://doi.org/10.3390/M1029
Chicago/Turabian StyleGonçalves, Itamar Luís, Luciano Porto Kagami, Gustavo Machado das Neves, Liliana Rockenbach, Leonardo Davi, Alceu Felipe Soares, Solange Cristina Garcia, and Vera Lucia Eifler-Lima. 2018. "Ethyl 4-(2-fluorophenyl)-6-methyl-2-thioxo-1-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate" Molbank 2018, no. 4: M1029. https://doi.org/10.3390/M1029
APA StyleGonçalves, I. L., Porto Kagami, L., Machado das Neves, G., Rockenbach, L., Davi, L., Soares, A. F., Garcia, S. C., & Eifler-Lima, V. L. (2018). Ethyl 4-(2-fluorophenyl)-6-methyl-2-thioxo-1-(p-tolyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank, 2018(4), M1029. https://doi.org/10.3390/M1029