Abstract
When 2-[(1S,2S)-1,2-diamino-2(2-hydroxyphenyl)ethyl]phenol (I) was reacted with 2 mole equivalents of trimethyl acetic (pivalic) anhydride in tetrahydrofuran at room temperature, 1-[(1S,2S)-1,2-bis(2-hydroxyphenyl)-2-pivaloylaminoethylamino]-2,2-dimethyl-1-propanone (II) was obtained quantitatively as the only product. The structure of the product was determined using 1H- and 13C-NMR. The COSY spectrum indicated that the single –NH was coupled to the single benzylic proton, –CH. The versality of the transformation could be used to generate additional compounds for use in various research areas.
1. Introduction
Amides and esters are important functional groups in organic chemistry and are distributed widely in naturally occurring compounds, including peptides in the case of amides and volatile components of fruits in the case of esters. Compounds containing these two functional groups are also routinely synthesized, and the two sources provide a variety of compounds for biological testing. While there are several methods for making amides and esters, the use of acyl chloride or acyl anhydride in a weak base like pyridine is usually employed, especially when the reactants are simple alcohols or amines [1]. Several other methods using mainly acetic anhydride with different bases and acids have been reported [2,3,4,5,6,7,8,9,10,11]. Acyl halides are generally more reactive and provide better atom efficiency than acyl anhydrides. When amine and alcohol groups are present in a compound and selective acylation is needed, it is important to consider the conditions and type of reagent that can lead to the acylation of a specific group and the less reactive acyl anhydride is a good choice. Usually, if the proper conditions are not used, both groups will be acylated, but in different amounts. Chemoselective acylation of amines in the presence of alcohols has been reported [12]. Pivalic anhydride, which was used in our study, has shown selectivity with hydroxy groups in carbohydrates even if the differences in the reactivity of those hydroxy groups are small [13,14,15,16]. The observed selectivity could be taken advantage of and used to preferentially react pivalic anhydride with –NH2 in the presence of –OH groups if the proper conditions are selected. Additionally, amines tend to be more nucleophilic than alcohols in acylation reactions, thus good amounts of amides can be obtained in the presence of alcohols. In this study, we selectively carried out the pivaloylation of the amino groups of 2-[(1S,2S)-1,2-diamino-2(2-hydroxyphenyl)ethyl]phenol (I), a compound with two amino and two hydroxy groups.
2. Results and Discussion
1-[(1S,2S)-1,2-Bis(2-hydroxyphenyl)-2-pivaloylaminoethylamino]-2,2-dimethyl-1-propanone (II) was synthesized using two mole equivalents of pivalic anhydride to one mole equivalent of compound I in tetrahydrofuran at room temperature. By adding pivalic anhydride to the amine dropwise, the selective acylation of the two amino groups was achieved. The structure of the product was determined using 1H and 13C-NMR. The 1H integration showed thirty-two protons and the 13C spectrum had ten types of carbon atoms, with C=O at 176.96 ppm, C-OH (phenolic) at 155.09 ppm, and CH3 at 27.73 ppm. COSY was used to confirm the structure by using the chemical shifts and coupling of –NH at 7.65 ppm with the characteristic benzylic –CH at 5.70 ppm.
While carrying out a literature search on the title compound, we found that compound I was selectively benzoylated at the –NH2 groups, however, no method of preparation, physical, or spectroscopic data were provided for the product. Here, we showed that it was possible to selectively and quantitatively react the amino groups of compound I with pivalic anhydride by using appropriate conditions including the solvent. This success presents opportunities to produce different amides and esters from a single compound, starting with different acid anhydrides.
3. Experimental
Tetrahydrofuran 99%, stabilized with 250–350 ppm BHT was obtained from Alfa Aesar (Thermo Fisher Scientific, Haverhill, MA, USA) and further dried using activated molecular sieves 3 Å. Trimethyl acetic (pivalic) anhydride and methanol were obtained from Sigma-Aldrich, St. Louis, MO, USA and used without further purification. 1H and 13C-NMR spectra were obtained using a Varian Gemini 400 NMR and recorded at 400 MHz and 100 MHz, respectively. Elemental analyses were performed by Robertson Microlit Laboratories Inc., Legdewood, NJ, USA.
Trimethyl acetic anhydride (1.86 g, 10.1 mmol) dissolved in 5 mL of THF was added dropwise to compound I (1.22 g, 5 mmol) dissolved in 4 mL of THF over a period of 2 min with stirring. Upon standing for 10 min, compound II precipitated from the reaction mixture, Scheme 1. The product was filtered, dried, and recrystallized from methanol to yield a pure compound as white needles (1.88 g, 91%, m.p. > 267 °C with decomposition).
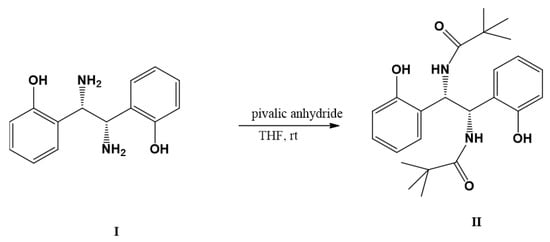
Scheme 1.
Synthesis of 1-[(1S,2S)-1,2-bis(2-hydroxyphenyl)-2-pivaloylaminoethylamino]-2,2-dimethyl-1-propanone (II).
1H-NMR (400 MHz, Me2SO-d6) δ 1.05 (s, 18H, –CH3), 5.70 (bs, 2H, –CH, benzylic), 6.25 (m, 2H, –aromatic), 6.60 (m, 2H, –aromatic), 6.85 (m, 2H, –aromatic), 7.00 (m, 2H, –aromatic), 7.65 (bs, 2H, –NH), 9.75 ( s, 2H, –OH). 13C-NMR (100 MHz, Me2SO-d6), δ 27.73 (–CH3), 38.48, 53.28, 115.5, 118.78, 126.76, 128.10, 129.27, 155.09 (–COH), 176.96 (C=O). C24H32N2O4 requires: C, 69.88%; H 7.82%; N 6.79%; O 15.51%. Found: C, 69.84%; H 7.58%, N 6.79%, O 15.82%
Supplementary Materials
The following are available online, Figure S1: 1H- and 13C-NMR spectra.
Funding
Funds from †he Pennsylvania State University-York Advisory Board Grant and Smith Funds were used for this work.
Conflicts of Interest
The author declares no conflict of interest.
References
- Olson, V.R.; Feldman, H. Quantitative Acetylation of Amines by Means of Acetyl Chloride and Pyridine. J. Am. Chem. Soc. 1937, 59, 2003–2005. [Google Scholar] [CrossRef]
- Romanelli, G.P.; Bennardi, D.O.; Autino, J.C.; Baronetti, G.T.; Thomas, H.J. A Simple and Mild Acylation of Alcohols, Phenols, Amine, and Thiols with a Reusable Heteropoly Acid Catalyst (H6P2W18O62·24H2O). E-J. Chem. 2008, 5, 641–647. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Gulhane, R.; Shivani. Copper (II) Tetrafluoroborate-Catalyzed Acetylation of Phenols, Thiols, Alcohols, and Amines. Synthesis 2004, 111–115. [Google Scholar] [CrossRef]
- Kadam, S.T.; Kim, S.S. Phosphomolybdic Acid: Mild and Efficient Catalyst for Acetylation of Alcohols, Phenols, and Amines under Solvent-Free Conditions. Synthesis 2008, 267–268. [Google Scholar] [CrossRef]
- Reddy, T.S.; Narasimhulu, M.; Suryakiran, N.; ChinniMahesh, K.; Ashalatha, K.; Venkateswarlu, Y. A mild and efficient acetylation of alcohols, phenols and amines with acetic anhydride using La(NO3)3·6H2O as a catalyst under solvent-free conditions. Tetr. Lett. 2006, 47, 6825–6829. [Google Scholar] [CrossRef]
- Kanta De, S. Ruthenium (III) chloride catalyzed acylation of alcohols, phenols, thiols, and amines. Tetr. Lett. 2004, 45, 2919–2922. [Google Scholar] [CrossRef]
- Das, B.; Thirupathi, P. A highly selective and efficient acetylation of alcohols and amines with acetic anhydride using NaHSO4·SiO2 as a heterogeneous catalyst. J. Mol. Catal. A Chem. 2007, 269, 12–16. [Google Scholar] [CrossRef]
- Aerry, S.; Kumar, A.; Saxena, A.; De, A.; Mozumdar, S. Chemoselective Acetylation of Amines and Thiols Using Monodispersed Ni-nanoparticles. Green Chem. Let. Rev. 2013, 6, 183–188. [Google Scholar] [CrossRef]
- Basu, K.; Chakraborty, S.; Sarkar, A.K.; Saha, C. Efficient Acetylation of Primary Amines and Amino acids in Environmentally Benign Solution Using Acetyl Chloride. J. Chem. Sci. 2013, 125, 607–613. [Google Scholar] [CrossRef]
- Qiu, R.; Zhu, Y.; Xu, X.; Li, Y.; Shao, L.; Ren, X.; Cai, X.; An, D.; Yin, S. Zirconocene bis(perfluorooctanesulfonate)s-catalyzed acylation of alcohols, phenols, thiols, and amines under solvent-free conditions. Catal. Commun. 2009, 10, 1889–1892. [Google Scholar] [CrossRef]
- Satam, J.R.; Jayaram, R.V. Acetylation of Alcohols, Phenols and Amines Using Ammonium Salt of 1,2-tungstophosphoric Acid: Environmentally Benign Method. Catal. Commun. 2008, 9, 2365–2370. [Google Scholar] [CrossRef]
- Naik, S.; Bhattacharjya, G.; Talukdar, B.; Patel, B.K. Chemoselective Acylation of Amines in Aqueous Media. Eur. J. Org. Chem. 2004, 1254–1260. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Denny, W.A.; Tercel, M.; Pruijn, F.B.; Ashoorzadeh, A. Nitro seco analogues of the duocarmycins containing sulfonate leaving groups as hypoxia-activated prodrugs for cancer therapy. J. Med. Chem. 2012, 55, 2780–2802. [Google Scholar] [CrossRef] [PubMed]
- Ljevaković, D.; Tomić, S.; Tomasić, J. Selective pivaloylation of 2-acetamido-2-deoxy sugars. Carbohyd. Res. 1988, 182, 197–205. [Google Scholar] [CrossRef]
- González, F.S.; García, J.I.; Berenguel, A.V.; Díaz, R.R.; Flores, F.G.C. Selective pivaloylation and diphenylacetylation of cyclomalto-oligosaccharides. Carbohyd. Res. 1994, 262, 271–282. [Google Scholar] [CrossRef]
- Babin, M.; Ruest, A.; Drouin, G.; Sirois, K.; Ouellet, S.; Gagno, J. Regioselective pivaloylation of N-phthaloylchitosan: A promising soluble intermediate for chitosan chemistry. Carbohyd. Res. 2012, 351, 87–92. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).