Abstract
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a cardioprotective phytochemical occurring in many plant products. In this study, a new series of imine congeners of resveratrol has been synthesized in which the imine moiety replaced the double bond in the structure of resveratrol. In addition, the in vitro antiplatelet activity of these resveratrol derivatives has been evaluated against adenosine diphosphate (ADP), arachidonic acid (AA), and collagen as platelet aggregation inducers. In general, the synthesized compounds were active as antiplatelet agents, and, therefore, the imine functional group may be considered as an effective replacement for a double bond in resveratrol for developing new and promising antiplatelet drugs.
1. Introduction
Cardiovascular diseases (CVDs) are recognized as the first global cause of death. It has been reported by the World Health Organization (WHO) that 17.7 million people died from CVDs in 2015 [1,2]. Platelet aggregation plays an essential role in the process of blood clotting and CVDs. However, many antiplatelet drugs such as aspirin and clopidogrel, which are available in clinics, are associated with some side effects such as bleeding and drug resistance that limit their usage [3,4]. Therefore, the search for new antiplatelet agents with fewer side effects and higher efficacy is among the priorities of medicinal chemists.
Natural products with various chemical structures have an important role in drug discovery and development [5,6,7,8,9]. Although chemical diversity of the natural products is pivotal in finding useful lead compounds, usually chemical modifications are needed to improve their potency and physicochemical properties [10,11,12]. Resveratrol (3,5,4′-trihydroxy-trans-stilbene) (1) (Figure 1) is one of these lead compounds. This stilbene is found in many natural sources such as grapes, apples, and berries [13,14,15]. Various biological activities of resveratrol have been reported such as anticancer, anti-inflammatory, antioxidant, and antiplatelet [13,16,17]. Orsini et al. synthesized and evaluated the antiplatelet aggregation activity of resveratrol 3-O-β-d-glucopyranoside and related hyroxystilbenes [18]. Dutra et al. synthesized new resveratrol and resveratrol-furoxan hybrids as antiplatelet and antithrombotic agents [19].
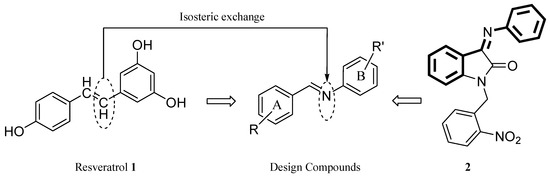
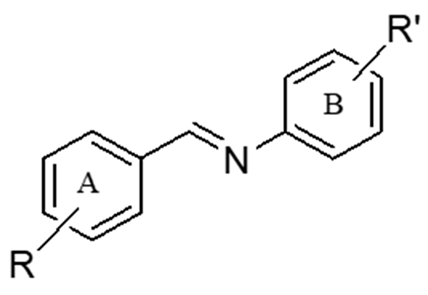
Figure 1.
Design of imine resveratrol derivatives.
A literature review revealed that C=N moiety is present in many structures with antiplatelet activity. Tehrani et al. synthesized a series of Schiff bases derived from 2-hydrazinyl-1,3,4-thiadiazole with high antiplatelet activity [20]. Akhlaghi et al. reported 3-(arylimino)indolin-2-one and 1-(aryl)-3-(phenylimino)indolin-2-one derivatives as antiplatelet agents [21]. Among their synthesized derivatives, compound (2) exhibited high antiplatelet activity against arachidonic acid (AA) as a platelet aggregation inducer (IC50 = 3.4 µM) [21]. Furthermore, antiplatelet activity of N′-benzylidene-carbohydrazide-1H-pyrazolo[3,4-b]pyridine derivatives have been reported [22]. A variety of indole hydrazone derivatives such as indole N-acylhydrazones [23,24], indole-3-carboxaldehyde phenylhydrazones [25], N-1 substituted indolehydrazones [26], indole-3-carbaldehyde, and indole-2-carbaldehayde hydrazones [27] have been previously synthesized in our research group. Some of these reported derivatives exhibited remarkable antiplatelet activity.
Therefore, the present research was aimed at the synthesis of a new series of imine congeners of resveratrol in which the imine moiety replaced the double bond in the structure of resveratrol (Figure 1) [28], and the evaluation of their in vitro antiplatelet activity against adenosine diphosphate (ADP), arachidonic acid (AA), and collagen as platelet aggregation inducers.
2. Results and Discussion
2.1. Chemistry
The designed compounds were synthesized by the reaction of different aniline derivatives with appropriate aldehydes in water as a green solvent without any catalyst (Figure 2). The synthesized derivatives (3a–3r) were obtained with high yields (>89%). Structure of the synthesized compounds was characterized by LC-MS 1H-NMR and 13C-NMR. The 1H-NMR spectra of the synthesized compounds exhibited a singlet peak for the CH=N proton between 8.51 and 8.96 ppm.
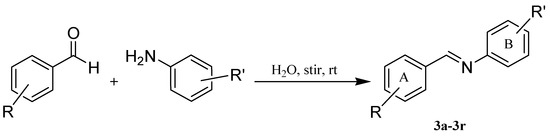
Figure 2.
Synthesis of resveratrol derivatives.
2.2. Anti-Platelet Activity
The anti-platelet activity of the synthesized derivatives against ADP, AA, and collagen as platelet aggregation inducers were evaluated, according to the Born method [29,30]. The obtained data are presented in Table 1.

Table 1.
Anti-platelet activity of the synthesized derivatives. Adenosine diphosphate (ADP), arachidonic acid (AA), and collagen were used as a platelet aggregation inducer at a final concentration of 5 µM, 1.35 µM, and 2.5 µg·mL−1, respectively. The results are expressed as the mean ± standard error of mean (SEM) from three independent experiments.
2.3. Structure Activity Relationship
The data reported in Table 1 show that all the compounds (3a–3r) at the concentration of 1 mM inhibited platelet aggregation induced by ADP, AA, and collagen. The inhibition range for ADP and collagen were 30%–84.6% and 15.9%–93.4%, respectively. When AA was used as a platelet aggregation inducer, the inhibition was increased and ranged from 74.7% to 100%.
Compound 3m with three-methoxy group on ring A inhibited platelet aggregation induced by all the three platelet inducers above 85%.
Since all compounds at concentration of 1 mM were able to completely inhibit platelet aggregation induced by AA, the IC50 values for these compounds were calculated (Table 1).
As shown in Table 1, all the compounds with hydroxyl substituent on the B ring show high activity (IC50 < 69.1 μM) except 3o. The results demonstrated that all the compounds with Schiff base and phenolic hydroxyl groups at the ortho position of ring A or B show IC50 values ranging between 19.8 μM and 30.7 μM except for 3c and 3p. Compounds 3i and 3q exhibited satisfactory activity with IC50 values of 29.9 μM and 30.7 μM, respectively. Compound 3r with IC50 value of 19.8 μM was the most active compound.
3. Materials and Methods
3.1. General Procedure for the Preparation of 3a–3r
The mixture of aromatic amine (1 mmol) and aldehyde (1 mmol) in water was stirred at room temperature. After completion of the reaction indicated by TLC (thin-layer chromatography), the obtained precipitate was filtered off and washed with water. The obtained precipitate was recrystallized from the appropriate solvent.
(E)-1-(4-Methoxyphenyl)-N-phenylmethanimine (3a). Yield 95%; m.p. 48–50 °C (m.p. 49–50 °C [31]). ESI-MS m/z: 212 [M + H]+. Anal. Calcd for C14H13NO: C 79.59, H 6.20, N 6.63, found C 79.57, H 6.21, N 6.64.
(E)-2-Methoxy-4-[(phenylimino)methyl]phenol (3b). Yield 89%. m.p. 159–162 °C (m.p. 158–160 °C [32]). ESI-MS m/z: 228 [M + H]+; Anal. Calcd for C14H13NO2: C 73.99, H 5.77, N 6.16, found C 73.97, H 5.76, N 6.15.
(E)-2-{[(4-Methoxyphenyl)imino]methyl}phenol (3c). Yield 94%. m.p. 76–77 °C; 1H-NMR (400 MHz, DMSO-d6) δ 13.32 (s, 1H, OH), 8.94 (s, 1H, HC=N), 7.61 (m, 1H, Ar-H), 7.42 (m, 3H, Ar-H), 6.96 (m, 4H, Ar-H), 3.79 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO-d6) δ 161.72, 160.62, 158.99, 141.19, 133.25, 132.80, 123.09, 119.85, 119.53, 116.97, 115.13, 55.86; ESI-MS m/z: 228 [M + H]+. Anal. Calcd for C14H13NO2: C 73.99, H 5.77, N 6.16, found C 73.95, H 5.76, N 6.17.
(E)-N,1-bis(4-Methoxyphenyl)methanimine (3d). Yield 89%. m.p. 154–155 °C (m.p. 154 °C [33]). ESI-MS m/z: 242 [M + H]+. Anal. Calcd for C15H15NO2: C 74.67, H 6.27, N 5.81, found C 74.68, H 6.28, N 5.79.
(E)-1-(3,4-Dimethoxyphenyl)-N-(4-methoxyphenyl)methanimine (3e). Yield 85%. m.p. 126–128 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.52 (s, 1H, HC=N), 6.96–7.54 (m, 7H, Ar-H), 3.77–3.83 (m, 9H, OCH3); 13C-NMR (100 MHz, DMSO-d6) δ 158.46, 158.05, 151.97, 149.45, 144.89, 129.77, 124.08, 122.66, 115.46, 114.95, 114.85, 111.74, 109.68, 56.07, 55.88, 55.73. ESI-MS m/z: 272 [M + H]+. Anal. Calcd for C16H17NO3: C 70.83, H 6.32, N 5.16, found C 70.81, H 6.31, N 5.17.
(E)-N-(4-Methoxyphenyl)-1-(p-tolyl)methanimine (3f). Yield 96%. m.p. 87–88 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.59 (s, 1H, HC=N), 7.70-7.81 (m, 2H, Ar-H), 7.31-7.42 (m, 4H, Ar-H), 6.98 (m, 2H, Ar-H), 3.78 (s, 3H, OCH3), 2.38 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO-d6) δ 158.2, 157.8, 144.2, 141.0, 133.8, 129.85, 128.88, 122.79, 114.87, 55.76, 21.1. ESI-MS m/z: 226 [M + H]+. Anal. Calcd for C15H15NO: C 79.97, H 6.71, N 6.22, found C 79.95, H 6.70, N 6.23.
(E)-2-Methoxy-4-{[(4-methoxyphenyl)imino]methyl}phenol (3g). Yield 94%. m.p. 154–155 °C (m.p. 154 °C [34]). ESI-MS m/z: 258 [M + H]+. Anal. Calcd for C15H15NO3: C 70.02, H 5.88, N 5.44, found C 70.01, H 5.89, N 5.43.
(E)-4-[(3-Methoxybenzylidene)amino]phenol (3h). Yield 94%. m.p. 165–167 °C (m.p. 167 °C [35]). ESI-MS m/z: 228 [M + H]+. Anal. Calcd for C14H13NO2: C 73.99, H 5.77, N 6.16, found C 73.97, H 5.76, N 6.15.
(E)-2-{[(4-Hydroxyphenyl)imino]methyl}phenol (3i). Yield 98%. m.p. 140–143 °C. 1H-NMR (400 MHz, DMSO-d6) δ 13.44 (s, 1H, OH), 9.72 (s, 1H, OH), 8.94 (s, 1H, HC=N), 7.58 (m, 1H, Ar-H), 7.31–7.37 (m, 3H, Ar-H), 6.84–6.95 (m, 4H, Ar-H). 13C-NMR (100 MHz, DMSO-d6) δ 160.67, 160.60, 157.41, 139.65, 133.00, 132.67, 123.12, 119.89, 119.46, 116.92, 116.43, 116.01, 115.85. ESI-MS m/z: 214 [M + H]+. Anal. Calcd for C13H11NO2: C 73.23, H 5.20, N 6.57, found C 73.21, H 5.21, N 6.56.
(E)-4-[(4-Methoxybenzylidene)amino]phenol (3j). Yield 95%. m.p. 187–188 °C (m.p. 189 °C [36]). ESI-MS m/z: 228 [M + H]+. Anal. Calcd for C14H13NO2: C 73.99, H 5.77, N 6.16, found C 74.20, H 5.77, N 6.15.
(E)-4-[(4-Methylbenzylidene)amino]phenol (3k). Yield 94%. m.p. 152–153 °C; 1H-NMR (400 MHz, DMSO-d6) δ 9.51 (s, 1H, OH), 8.55 (s, 1H, HC=N), 7.78 (m, 2H, Ar-H), 7.18–7.30 (m, 4H, Ar-H), 6.80 (m, 2H, Ar-H), 2.36 (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ 157.51, 156.57, 143.19, 141.24, 134.36, 129.82, 128.73, 122.86, 116.15, 21.59; ESI-MS m/z: 212 [M + H]+. Anal. Calcd for C14H13NO: C 79.59, H 6.20, N 6.63, found C 79.45, H 6.19, N 6.62.
(E)-4-[(3,4-Dimethoxybenzylidene)amino]phenol (3l). Yield 92%. m.p. 155–156 °C (m.p. 155 °C [37]). ESI-MS m/z: 258 [M + H]+. Anal. Calcd for C15H15NO3: C 70.02, H 5.88, N 5.44, found C 70.10, H 5.87, N 5.45.
(E)-4-[(3,4,5-Trimethoxybenzylidene)amino]phenol (3m). Yield 96%. m.p. 140–142 °C. 1H-NMR (400 MHz, DMSO-d6) δ 9.53 (s, 1H, OH), 8.51 (s, 1H, HC=N), 7.18–7.23 (m, 4H, Ar-H), 6.81 (m, 2H, Ar-H), 3.85 (s, 6H, OCH3), 3.73 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO-d6) δ 157.42, 156.60, 153.58, 143.06, 140.31, 132.44, 122.85, 116.19, 115.87, 107.19, 105.88, 60.61, 56.50, 56.36; ESI-MS m/z: 288 [M + H]+. Anal. Calcd for C16H17NO4: C 66.89, H 5.96, N 4.88, found C 66.84, H 5.95, N 4.88.
(E)-3-{[(4-Hydroxyphenyl)imino]methyl}phenol (3n). Yield 94%. m.p. 191–193 °C. 1H-NMR (400 MHz, DMSO-d6) δ 9.69 (s, 1H, OH), 9.55 (s, 1H, OH), 8.51 (s, 1H, HC=N), 7.19–7.37 (m, 5H, Ar-H), 6.83–6.91 (m, 3H, Ar-H). 13C-NMR (100 MHz, DMSO-d6) δ 158.10, 157.66, 156.70, 143.04, 138.29, 130.23, 122.95, 120.44, 118.64, 116.19, 115.90, 114.35; ESI-MS m/z: 214 [M + H]+. Anal. Calcd for C13H11NO2: C 73.23, H 5.20, N 6.57, found C 73.21, H 5.21, N 6.58.
(E)-4-[(2-Methoxybenzylidene)amino]phenol (3o). Yield 92%. m.p. 168–169 °C (m.p. 168 °C [35]). ESI-MS m/z: 228 [M + H]+. Anal. Calcd for C14H13NO2: C 73.99, H 5.77, N 6.16, found C 73.94, H 5.78, N 6.15.
(E)-2-[(2-Hydroxybenzylidene)amino]phenol (3p). Yield 98%. m.p. 141–143 °C. 1H-NMR (400 MHz, DMSO-d6) δ 13.81 (s, 1H, OH), 9.77 (s, 1H, OH), 8.96 (s, 1H, HC=N), 7.13–7.61 (m, 4H, Ar-H), 6.89–6.95 (m, 4H, Ar-H). 13C-NMR (100 MHz, DMSO-d6) δ 162.13, 161.20, 151.56, 135.40, 133.31, 132.78, 128.53, 120.09, 120.04, 119.96, 119.21, 117.16, 116.98. ESI-MS m/z: 214 [M + H]+. Anal. Calcd for C13H11NO2: C 73.23, H 5.20, N 6.57, found C 73.24, H 5.19, N 6.56.
(E)-2-[(3-Hydroxybenzylidene)amino]phenol (3q). Yield 99%. m.p. 122–124 °C (m.p. 122.5–123.5 °C [38]). ESI-MS m/z: 214 [M + H]+. Anal. Calcd for C13H11NO2: C 73.23, H 5.20, N 6.57, found C 73.24, H 5.19, N 6.55.
(E)-2-[(4-Methylbenzylidene)amino]phenol (3r). Yield 98%. m.p. 107–108 °C (m.p. 108.5 °C [39]). ESI-MS m/z: 212 [M + H]+. Anal. Calcd for C14H13NO: C 79.59, H 6.20, N 6.63, found C 79.58, H 6.19, N 6.64.
3.2. Anti-Platelet Assay
The anti-platelet aggregation activity of the synthesized compounds was evaluated on an APACT 4004 aggregometer (LABiTec, Ahrensburg, Germany), according to the method described before [29,40,41]. Compounds (3a–3r) were added to platelet-rich plasma (PRP) and were incubated for 5 min at 37 °C. Adenosine diphosphate (ADP), arachidonic acid, and collagen were added separately as platelet aggregation inducers at a final concentration of 5 µM, 1.35 µM, and 2.5 µg·mL−1, respectively. The aggregation procedure was monitored for 5 min. Compounds were screened at a concentration of 1 mM in DMSO. The IC50 values against AA were determined for the synthesized compounds. Each experiment was carried out in triplicate and the results are shown as a mean ± standard error of mean (SEM).
4. Conclusions
In the present study, a series of resveratrol derivatives was synthesized and their antiplatelet activity was evaluated against ADP, AA, and collagen as platelet aggregation inducers. Compound 3r was the most active agent against AA and, therefore, possesses the potential to be considered a lead compound for future studies and further investigations. Lastly, the imine functional group may be qualified as an effective replacement for the double bond in resveratrol for anti-platelet aggregation pharmacophore.
Supplementary Materials
The following are available online http://www.mdpi.com/1422-8599/2019/1/M1039/s1, Figure S1: Qualitative data analysis report.
Author Contributions
M.B., M.S., M.E., and F.K. carried out the experiments, analyzed the results, and wrote the manuscript. S.V., M.I., and J.S.-R. contributed to the discussion of results and critically reviewed the manuscript. All the authors read and approved the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Organization, W.H. Cardiovascular Diseases. Fact Sheet 317. 2007. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 May 2017).
- Palmer, M.; Sutherland, J.; Barnard, S.; Wynne, A.; Rezel, E.; Doel, A.; Grigsby-Duffy, L.; Edwards, S.; Russell, S.; Hotopf, E. The effectiveness of smoking cessation, physical activity/diet and alcohol reduction interventions delivered by mobile phones for the prevention of non-communicable diseases: A systematic review of randomised controlled trials. PLoS ONE 2018, 13, e0189801. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Patel, K.; Crane, T. A review of antiplatelet drugs, coronary artery diseases and cardiopulmonary bypass. J. Extr.-Corporeal Technol. 2010, 42, 103. [Google Scholar]
- Guthrie, R. Review and management of side effects associated with antiplatelet therapy for prevention of recurrent cerebrovascular events. Adv. Ther. 2011, 28, 473. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Fokou, P.; Sharopov, F.; Martorell, M.; Ademiluyi, A.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Albayrak, S.; Antolak, H.; Kręgiel, D.; Pawlikowska, E.; Sharifi-Rad, M.; Uprety, Y.; Tsouh Fokou, P.; Yousef, Z.; Amiruddin Zakaria, Z. Aloe genus plants: From farm to food applications and phytopharmacotherapy. Int. J. Mol. Sci. 2018, 19, 2843. [Google Scholar] [CrossRef] [PubMed]
- Prakash, M.A.; Sharifi-Rad, M.; Shariati, M.; Mabkhot, Y.; Al-Showiman, S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P. Bioactive compounds and health benefits of edible Rumex species-a review. Cell. Mol. Biol. (Noisy-le-Grand, France) 2018, 64, 27. [Google Scholar] [CrossRef]
- Mishra, A.; Saklani, S.; Salehi, B.; Parcha, V.; Sharifi-Rad, M.; Milella, L.; Iriti, M.; Sharifi-Rad, J.; Srivastava, M. Satyrium nepalense, a high altitude medicinal orchid of indian himalayan region: Chemical profile and biological activities of tuber extracts. Cell. Mol. Biol. (Noisy-le-Grand, France) 2018, 64, 35–43. [Google Scholar] [CrossRef]
- Guo, Z. The modification of natural products for medical use. Acta Pharm. Sinica B 2017, 7, 119–136. [Google Scholar] [CrossRef]
- Bovicelli, P.; Bernini, R.; Antonioletti, R.; Mincione, E. Selective halogenation of flavanones. Tetrahedron Lett. 2002, 43, 5563–5567. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kręgiel, D.; Mileski, K.S.; Sharifi-Rad, M. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Olas, B.; Wachowicz, B. Resveratrol, a phenolic antioxidant with effects on blood platelet functions. Platelets 2005, 16, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Wang, X.-B.; Kong, L.-Y. Design, synthesis and biological evaluation of imine resveratrol derivatives as multi-targeted agents against Alzheimer’s disease. Eur. J. Med. Chem. 2014, 71, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Vitalini, S. Chemical diversity of grape products, a complex blend of bioactive secondary metabolites. Nat. Prod. J. 2011, 1, 71–74. [Google Scholar]
- Shen, M.Y.; Hsiao, G.; Liu, C.L.; Fong, T.H.; Lin, K.H.; Chou, D.S.; Sheu, J.R. Inhibitory mechanisms of resveratrol in platelet activation: Pivotal roles of p38 mapk and no/cyclic gmp. Br. J. Haematology 2007, 139, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer molecular mechanisms of resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Pelizzoni, F.; Verotta, L.; Aburjai, T.; Rogers, C.B. Isolation, synthesis, and antiplatelet aggregation activity of resveratrol 3-O-β-d-glucopyranoside and related compounds. J. Nat. Prod. 1997, 60, 1082–1087. [Google Scholar] [CrossRef]
- Dutra, L.A.; Guanaes, J.F.O.; Johmann, N.; Pires, M.E.L.; Chin, C.M.; Marcondes, S.; Dos Santos, J.L. Synthesis, antiplatelet and antithrombotic activities of resveratrol derivatives with no-donor properties. Bioorg. Med. Chem. Lett. 2017, 27, 2450–2453. [Google Scholar] [CrossRef]
- Tehrani, K.H.M.E.; Sardari, S.; Mashayekhi, V.; Zadeh, M.E.; Azerang, P.; Kobarfard, F. One pot synthesis and biological activity evaluation of novel schiff bases derived from 2-hydrazinyl-1,3,4-thiadiazole. Chem. Pharm. Bull. 2013, 61, 160–166. [Google Scholar] [CrossRef]
- Akhlaghi, M.F.; Amidi, S.; Esfahanizadeh, M.; Daeihamed, M.; Kobarfard, F. Synthesis of n-arylmethyl substituted indole derivatives as new antiplatelet aggregation agents. Iranian J. Pharm. Res. 2014, 13, 35. [Google Scholar]
- Lourenço, A.L.; Salvador, R.R.; Silva, L.A.; Saito, M.S.; Mello, J.F.; Cabral, L.M.; Rodrigues, C.R.; Vera, M.A.; Muri, E.M.; de Souza, A.M. Synthesis and mechanistic evaluation of novel N′-benzylidene-carbohydrazide-1H-pyrazolo [3,4-b] pyridine derivatives as non-anionic antiplatelet agents. Eur. J. Med. Chem. 2017, 135, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Mirfazli, S.S.; Kobarfard, F.; Firoozpour, L.; Asadipour, A.; Esfahanizadeh, M.; Tabib, K.; Shafiee, A.; Foroumadi, A. N-substituted indole carbohydrazide derivatives: Synthesis and evaluation of their antiplatelet aggregation activity. DARU J. Pharm. Sci. 2014, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Mirfazli, S.S.; Khoshneviszadeh, M.; Jeiroudi, M.; Foroumadi, A.; Kobarfard, F.; Shafiee, A. Design, synthesis and qsar study of arylidene indoles as anti-platelet aggregation inhibitors. Med. Chem. Res. 2016, 25, 1–18. [Google Scholar] [CrossRef]
- Tehrani, K.H.M.E.; Zadeh, M.E.; Mashayekhi, V.; Hashemi, M.; Kobarfard, F.; Gharebaghi, F.; Mohebbi, S. Synthesis, antiplatelet activity and cytotoxicity assessment of indole-based hydrazone derivatives. Iranian J. Pharm. Res. 2015, 14, 1077. [Google Scholar]
- Kalhor, N.; Mardani, M.; Abdollahzadeh, S.; Vakof, M.; Zadeh, M.E.; Tehrani, K.H.M.E.; Kobarfard, F.; Mohebbi, S. Novel N-substituted ((1H-indol-3-yl)methylene)benzohydrazides and ((1H-indol-3-yl)methylene)-2-phenylhydrazines: Synthesis and antiplatelet aggregation activity. Bull. Korean Chem. Soc. 2015, 36, 2632–2639. [Google Scholar] [CrossRef]
- Mashayekhi, V.; Tehrani, K.H.M.E.; Amidi, S.; Kobarfard, F. Synthesis of novel indole hydrazone derivatives and evaluation of their antiplatelet aggregation activity. Chem. Pharm. Bull. 2013, 61, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.; Barreiro, E.J. Bioisosterism: A useful strategy for molecular modification and drug design. Curr. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Amidi, S.; Kobarfard, F.; Moghaddam, A.B.; Tabib, K.; Soleymani, Z. Electrochemical synthesis of novel 1, 3-indandione derivatives and evaluation of their antiplatelet aggregation activities. Iranian J. Pharm. Res. 2013, 12, 91. [Google Scholar]
- Born, G.V.R. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef]
- Iovel, I.; Golomba, L.; Fleisher, M.; Popelis, J.; Grinberga, S.; Lukevics, E. Hydrosilylation of (hetero) aromatic aldimines in the presence of a Pd (I) complex. Chem. Heterocycl. Compd. 2004, 40, 701–714. [Google Scholar] [CrossRef]
- Cheng, L.-X.; Tang, J.-J.; Luo, H.; Jin, X.-L.; Dai, F.; Yang, J.; Qian, Y.-P.; Li, X.-Z.; Zhou, B. Antioxidant and antiproliferative activities of hydroxyl-substituted schiff bases. Bioorg. Med. Chem. Lett. 2010, 20, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.; Hassan, A.; Ibraheem, N.; Hekal, B. Synthesis and comparative studies of cyclopalladated complexes with ortho c‒h activation of aromatic rings bearing electron donating and electron withdrawing groups. Synth. React. Inorg. Met.-Org. Nano-Metal Chem. 2015, 45, 813–820. [Google Scholar] [CrossRef]
- Gebretekle, D.; Tadesse, A.; Upadhyay, R.; Dekebo, A. Synthesis, characterization and antimicrobial evaluation of some schiff bases and their thiazolidinone products. Oriental J. Chem. 2012, 28, 1791–1796. [Google Scholar] [CrossRef]
- Grammaticakis, P.; Texier, H. Contribution al’étude de l’absorption dans l’ultraviolet moyen et le visible de derivés fonctionnels azotés de quelques aldéhydes et cétones aromatiques. X.—aniles (premier mémoire). Bull. Soc. Chim. Fr. 1971, 38, 1323–1330. [Google Scholar]
- Oliveira Calil, N.; Senra Goncalves de Carvalho, G.; Farah da Silva, A.; David da Silva, A.; Rezende Barbosa Raposo, N. Antioxidant activity of synthetic resveratrol analogs: A structure-activity insight. Lett. Drug Des. Discovery 2012, 9, 676–679. [Google Scholar] [CrossRef]
- Ceyhan, G.; Köse, M.; Tümer, M.; Demirtaş, İ. Anticancer, photoluminescence and electrochemical properties of structurally characterized two imine derivatives. Spectrochim. Acta Part A Mol. Biomolecular. Spectrosc. 2015, 149, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Kazantsev, A.V.; Aksartov, M.M.; Zakharkin, L.I. 1,4-Addition der lithium-und magnesiumderivate von o-carboranen an alpha, beta-nitroolefine. Zh. Obshch. Khim. [J. Gen. Chem. USSR (Engl. Transl.)] 1971, 41, 57. [Google Scholar]
- Singleton, F.; Pollard, C. Reactions of aldehydes with amines. I. With o-aminophenol. J. Am. Chem. Soc. 1940, 62, 2288–2289. [Google Scholar] [CrossRef]
- Lorigooini, Z.; Ayatollahi, S.A.; Amidi, S.; Kobarfard, F. Evaluation of anti-platelet aggregation effect of some Allium species. Iranian J. Pharm. Res. 2015, 14, 1225. [Google Scholar]
- Amidi, S.; Esfahanizadeh, M.; Tabib, K.; Soleimani, Z.; Kobarfard, F. Rational design and synthesis of 1-(arylideneamino)-4-aryl-1H-imidazole-2-amine derivatives as antiplatelet agents. Chem. Med. Chem. 2017, 12, 962–971. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).