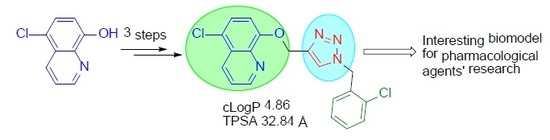
5-Chloro-8-{[1-(2-chlorobenzyl)-1H-1,2,3-triazol-4-yl]methoxy}quinoline
Abstract
:1. Introduction
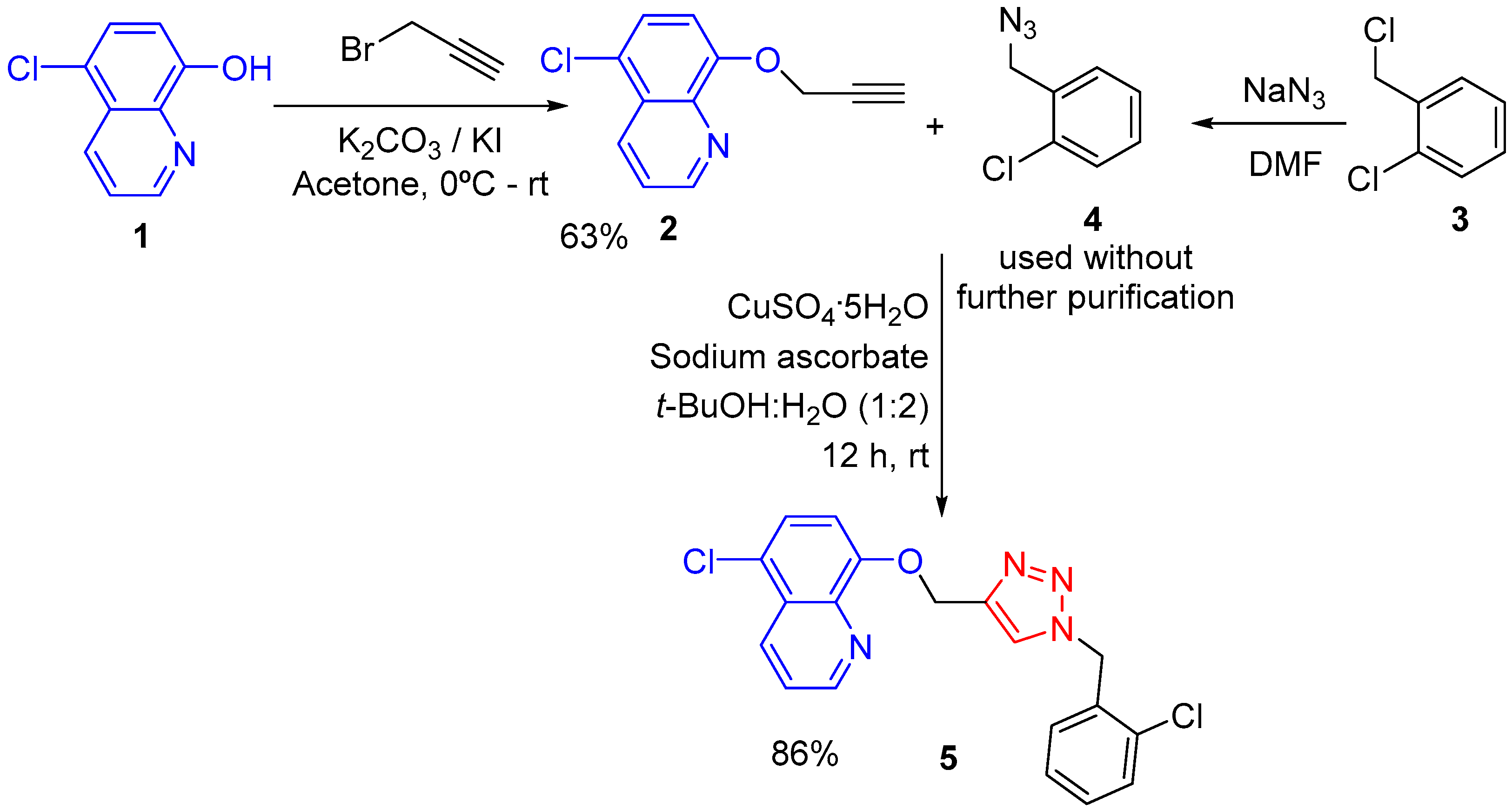
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Analysis
3.2. Synthesis of 5-Chloro-8-{[1-(2-chlorobenzyl)-1H-1,2,3-triazol-4-yl]methoxy}quinoline (6)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marella, A.; Tanwar, O.P.; Saha, R.; Ali, M.R.; Srivastava, S.; Akhter, M.; Shaquiquzzaman, M.; Alam, M.M. Quinoline: A versatile heterocyclic. Saudi Pharm. J. 2013, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jagg, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kavitha, P.H. Synthesis and biological applications of triazole derivatives-a review. Mini-Rev. Med. Chem. 2013, 10, 40–65. [Google Scholar]
- Bohm, R.; Karow, C. Biologically active triazoles. Pharmazie 1981, 36, 243–247. [Google Scholar] [PubMed]
- Luna-Parada, L.K.; Vargas-Méndez, L.Y.; Kouznetsov, V.V. Quinoline-Substituted 1,2,3-Triazole-Based Molecules, As Promising Conjugated Hybrids in Biomedical Research. Org. Med. Chem. J. 2018, 7. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Vargas-Méndez, L.Y.; Zubkov, F.I. Recent Advances in Synthesis of Bioactive Quinoline-based 1,2,3-Triazoles via Cu-catalyzed Huisgen 1,3-Dipolar Cycloaddition (“Click reaction”). Mini-Rev. Org. Chem. 2016, 13, 488–503. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Totobenazara, J.; Burke, A.J. New click-chemistry methods for 1,2,3-triazoles synthesis: Recent advances and applications. Tetrahedron Lett. 2015, 56, 2853–2859. [Google Scholar] [CrossRef]
- Irfan, M.; Alam, S.; Manzoor, N.; Abid, M. Effect of quinoline based 1,2,3-triazole and its structural analogues on growth and virulence attributes of Candida albicans. PLoS ONE 2017, 12, e0175710. [Google Scholar] [CrossRef] [PubMed]
- De O. Freitas, L.B.; Borgati, T.F.; de Freitas, R.P.; Ruiz, A.L.T.G.; Marchetti, G.M.; de Carvalho, J.E.; da Cunha, E.F.F.; Ramalho, T.C.; Alves, R.B. Synthesis and antiproliferative activity of 8-hydroxyquinoline derivatives containing a 1,2,3-triazole moiety. Eur. J. Med. Chem. 2014, 84, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, R.B.; Rindhe, S.S. Synthesis and antimicrobial activities of novel n-substituted 8-(1-alkyl/alkylsulphonyl/alkoxycarbonyl-benzimidazol-2-ylmethoxy)-5-chloroquinolines. J. Serb. Chem. Soc. 2011, 76, 1199–1206. [Google Scholar] [CrossRef]
- Kaur, K.; Jain, M.; Reddy, R.P.; Jain, R. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010, 45, 3245–3264. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, V.; Vecchio, G. 8-Hydroxyquinolines in medicinal chemistry: A structural perspective. Eur. J. Med. Chem. 2016, 120, 252–274. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar]
- Molinspiration Cheminformatics. Available online: http://www.molinspiration.com. (accessed on 12 November 2018).
- Veber, D.F.; Jhonson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna Parada, L.K.; Kouznetsov, V.V. 5-Chloro-8-{[1-(2-chlorobenzyl)-1H-1,2,3-triazol-4-yl]methoxy}quinoline. Molbank 2019, 2019, M1038. https://doi.org/10.3390/M1038
Luna Parada LK, Kouznetsov VV. 5-Chloro-8-{[1-(2-chlorobenzyl)-1H-1,2,3-triazol-4-yl]methoxy}quinoline. Molbank. 2019; 2019(1):M1038. https://doi.org/10.3390/M1038
Chicago/Turabian StyleLuna Parada, Luz Karime, and Vladimir V. Kouznetsov. 2019. "5-Chloro-8-{[1-(2-chlorobenzyl)-1H-1,2,3-triazol-4-yl]methoxy}quinoline" Molbank 2019, no. 1: M1038. https://doi.org/10.3390/M1038
APA StyleLuna Parada, L. K., & Kouznetsov, V. V. (2019). 5-Chloro-8-{[1-(2-chlorobenzyl)-1H-1,2,3-triazol-4-yl]methoxy}quinoline. Molbank, 2019(1), M1038. https://doi.org/10.3390/M1038