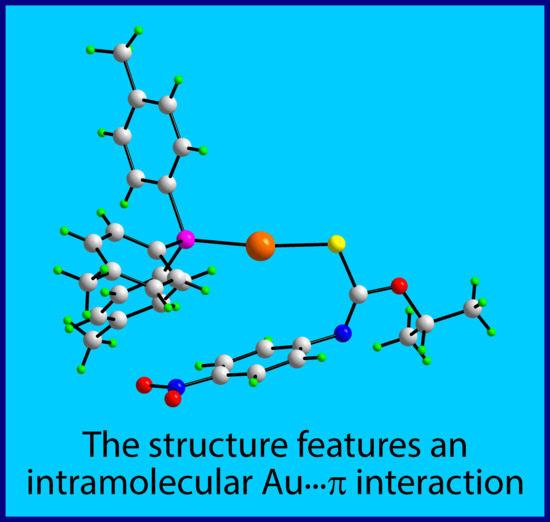
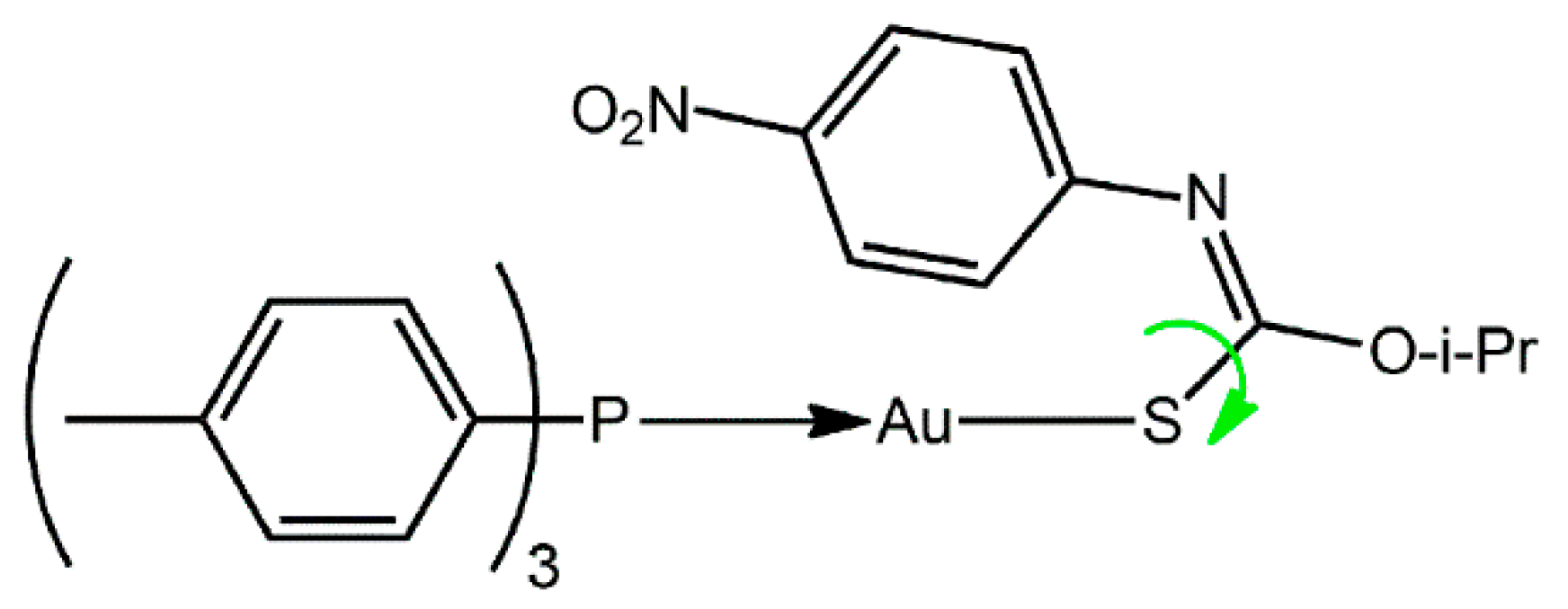
[O-Isopropyl-N-(4-nitrophenyl)thiocarbamato-κS]-(tri-4-tolylphosphine-κP)gold(I)
Abstract
1. Introduction
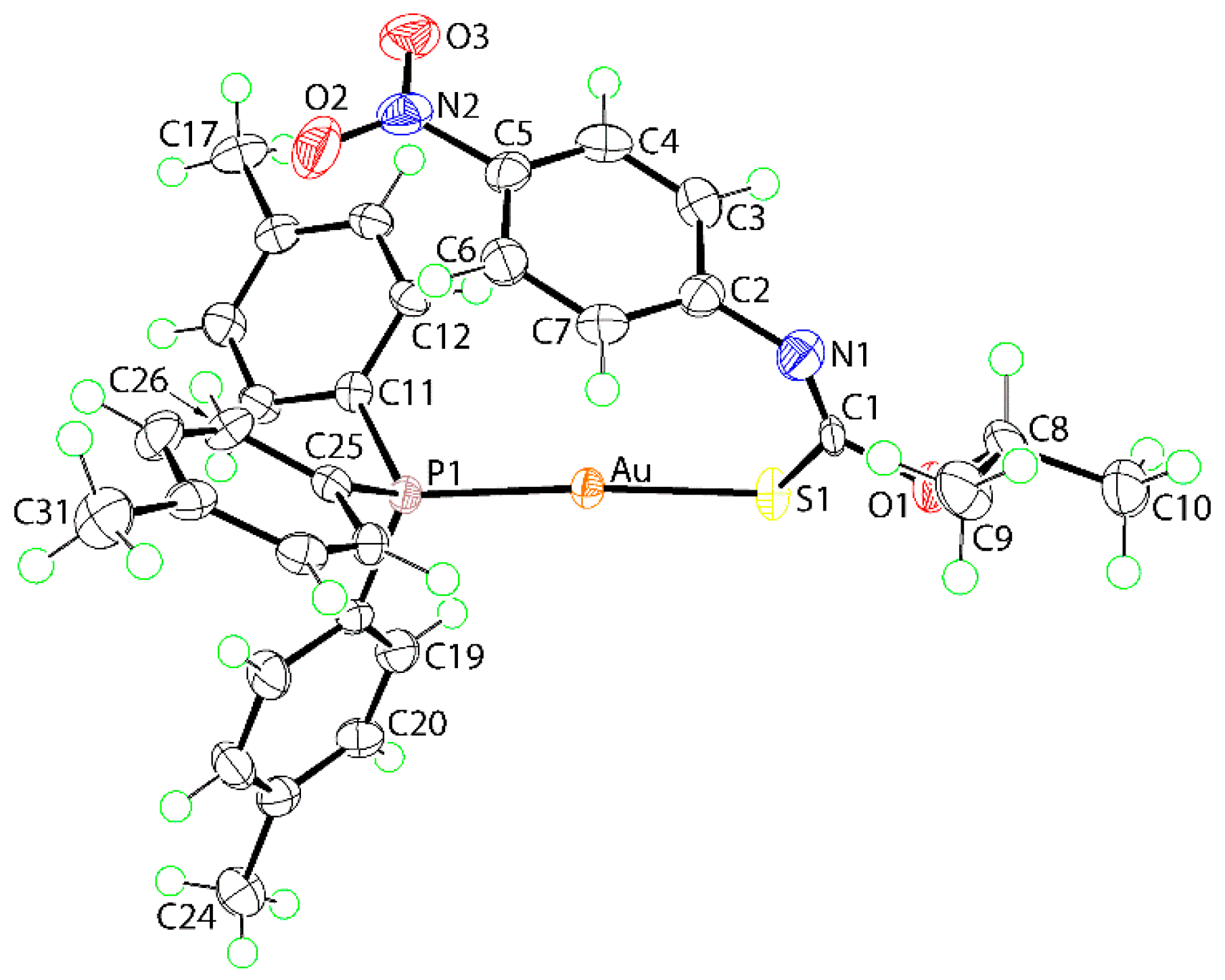
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization
3.3. X-ray Crystallography
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haruta, M. Gold as a novel catalyst in the 21st century: Preparation, working mechanism and applications. Gold Bull. 2004, 37, 27–36. [Google Scholar] [CrossRef]
- Rudolph, M.; Hashmi, A.S.K. Gold catalysis in total synthesis—An update. Chem. Soc. Rev. 2012, 41, 2448–2462. [Google Scholar] [CrossRef] [PubMed]
- Tiekink, E.R.T.; Zukerman-Schpector, J. Gold…π aryl interactions as supramolecular synthons. CrystEngComm 2009, 11, 1176–1186. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Zukerman-Schpector, J. A structural survey of metal/π heteroaromatic supramolecular synthons for metal = tellurium, tin, and gold. CrystEngComm 2009, 11, 2701–2711. [Google Scholar] [CrossRef]
- Caracelli, I.; Zukerman-Schpector, J.; Tiekink, E.R.T. Supra-molecular synthons based on gold…π(arene) interactions. Gold Bull. 2013, 46, 81–89. [Google Scholar] [CrossRef]
- Yeo, C.I.; Khoo, C.-H.; Chu, W.-C.; Chen, B.-J.; Chu, P.-L.; Sim, J.-H.; Cheah, Y.-K.; Ahmad, J.; Halim, S.N.A.; Seng, H.-L.; et al. The importance of Au…π(aryl) interactions in the formation of spherical aggregates in binuclear phosphanegold(I) complexes of a bipodal thiocarbamate dianion: A combined crystallographic and computational study, and anti-microbial activity. RSC Adv. 2015, 5, 41401–41411. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Supramolecular assembly of molecular gold(I) compounds: An evaluation of the competition and complementarity between aurophilic (Au…Au) and conventional hydrogen bonding interactions. Coord. Chem. Rev. 2014, 275, 130–153. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Supramolecular assembly based on “emerging” intermolecular interactions of particular interest to coordination chemists. Coord. Chem. Rev. 2017, 345, 209–228. [Google Scholar] [CrossRef]
- Yeo, C.-I.; Tan, S.L.; Otero-de-la-Roza, A.; Tiekink, E.R.T. A conformational polymorph of Ph3PAu[SC(OEt)=NPh] featuring an intramolecular Au…π interaction. Z. Kristallogr. Cryst. Mater. 2016, 231, 653–661. [Google Scholar] [CrossRef]
- Hall, V.J.; Tiekink, E.R.T. Crystal structure of triphenylphosphine-(N-phenyl-O-ethylthiocarbamato)gold(I), (C6H5)3PAu(SC(=NPh)OEt). Z. Kristallogr. 1993, 203, 313–315. [Google Scholar] [CrossRef]
- Kuan, F.S.; Ho, S.Y.; Tadbuppa, P.P.; Tiekink, E.R.T. Electronic and steric control over Au…Au, C-H…O and C-H…interactions in the crystal structures of mononuclear triarylphosphinegold(I) carbonimidothioates: R3PAu[SC(OMe)=NR′] for R = Ph, o-tol, m-tol or p-tol, and R′ = Ph, o-tol, m-tol, p-tol or C6H4NO2-4. CrystEngComm 2008, 10, 548–564. [Google Scholar] [CrossRef]
- Ellis, C.A.; Tiekink, E.R.T.; Zukerman-Schpector, J. (E)-O-Isopropyl N-(4-nitrophenyl)thiocarbamate. Acta Crystallogr. E 2008, 64, o345. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Cheng, E.C.-C.; Tiekink, E.R.T.; Yam, V.W.-W. Luminescent phosphine gold(I) thiolates: Correlation between crystal structure and photoluminescent properties in [R3PAu{SC(OMe)=NC6H4NO2-4}] (R = Et, Cy, Ph) and [(Ph2P-R-PPh2){AuSC(OMe)=NC6H4NO2-4}2] (R = CH2, (CH2)2, (CH2)3, (CH2)4, Fc). Inorg. Chem. 2006, 45, 8165–8174. [Google Scholar] [CrossRef] [PubMed]
- Schollmeyer, D.; Shishkin, O.V.; Rühl, T.; Vysotsky, M.O. OH–π and halogen–π interactions as driving forces in the crystal organisations of tri-bromo and tri-iodo trityl alcohols. CrystEngComm 2008, 10, 715–723. [Google Scholar] [CrossRef]
- Shishkin, O.V. Evaluation of true energy of halogen bonding in the crystals of halogen derivatives of trityl alcohol. Chem. Phys. Lett. 2008, 458, 96–100. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Janiak, J. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction, CrysAlis PRO; Agilent Technologies Inc.: Santa Clara, CA, USA, 2017.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
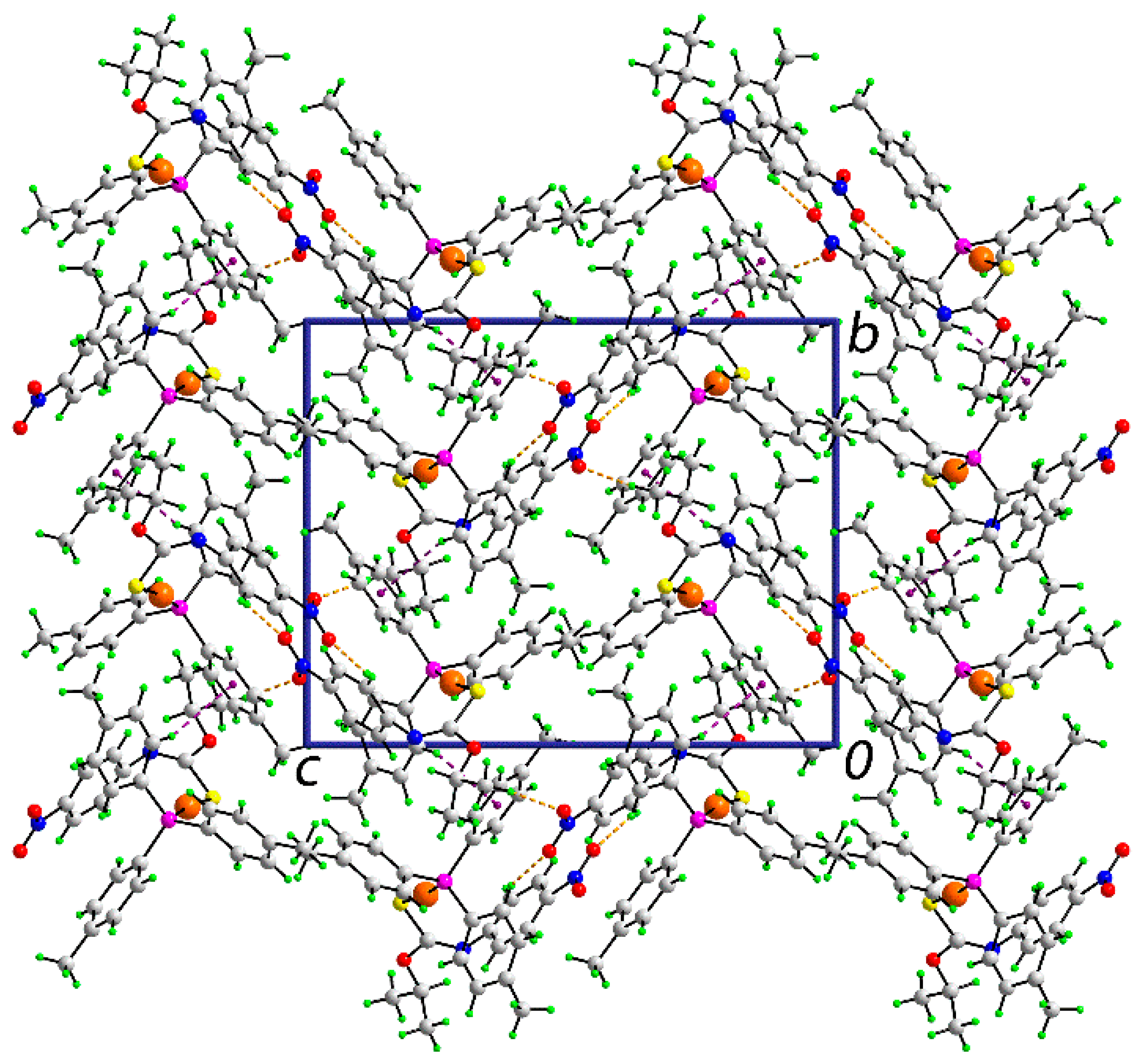
| A | H | B | H···B | A···B | A–H···B | Symmetry Operation |
|---|---|---|---|---|---|---|
| C10 | H10a | O2 | 2.43 | 3.382(7) | 165 | ½ − x, 1 − y, ½ + z |
| C26 | H26 | O3 | 2.46 | 3.194(7) | 134 | ½ + x, 1½ − y, 1 − z |
| C30 | H30 | Cg(C11–C16) | 2.97 | 3.833(5) | 152 | 1 − x, −½ + y, 1½ − z |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, C.I.; Tiekink, E.R.T. [O-Isopropyl-N-(4-nitrophenyl)thiocarbamato-κS]-(tri-4-tolylphosphine-κP)gold(I). Molbank 2018, 2018, M1028. https://doi.org/10.3390/M1028
Yeo CI, Tiekink ERT. [O-Isopropyl-N-(4-nitrophenyl)thiocarbamato-κS]-(tri-4-tolylphosphine-κP)gold(I). Molbank. 2018; 2018(4):M1028. https://doi.org/10.3390/M1028
Chicago/Turabian StyleYeo, Chien Ing, and Edward R. T. Tiekink. 2018. "[O-Isopropyl-N-(4-nitrophenyl)thiocarbamato-κS]-(tri-4-tolylphosphine-κP)gold(I)" Molbank 2018, no. 4: M1028. https://doi.org/10.3390/M1028
APA StyleYeo, C. I., & Tiekink, E. R. T. (2018). [O-Isopropyl-N-(4-nitrophenyl)thiocarbamato-κS]-(tri-4-tolylphosphine-κP)gold(I). Molbank, 2018(4), M1028. https://doi.org/10.3390/M1028