(S)-4-Isopropyl-5,5-diphenyloxazolidin-2-one
Abstract
:1. Introduction
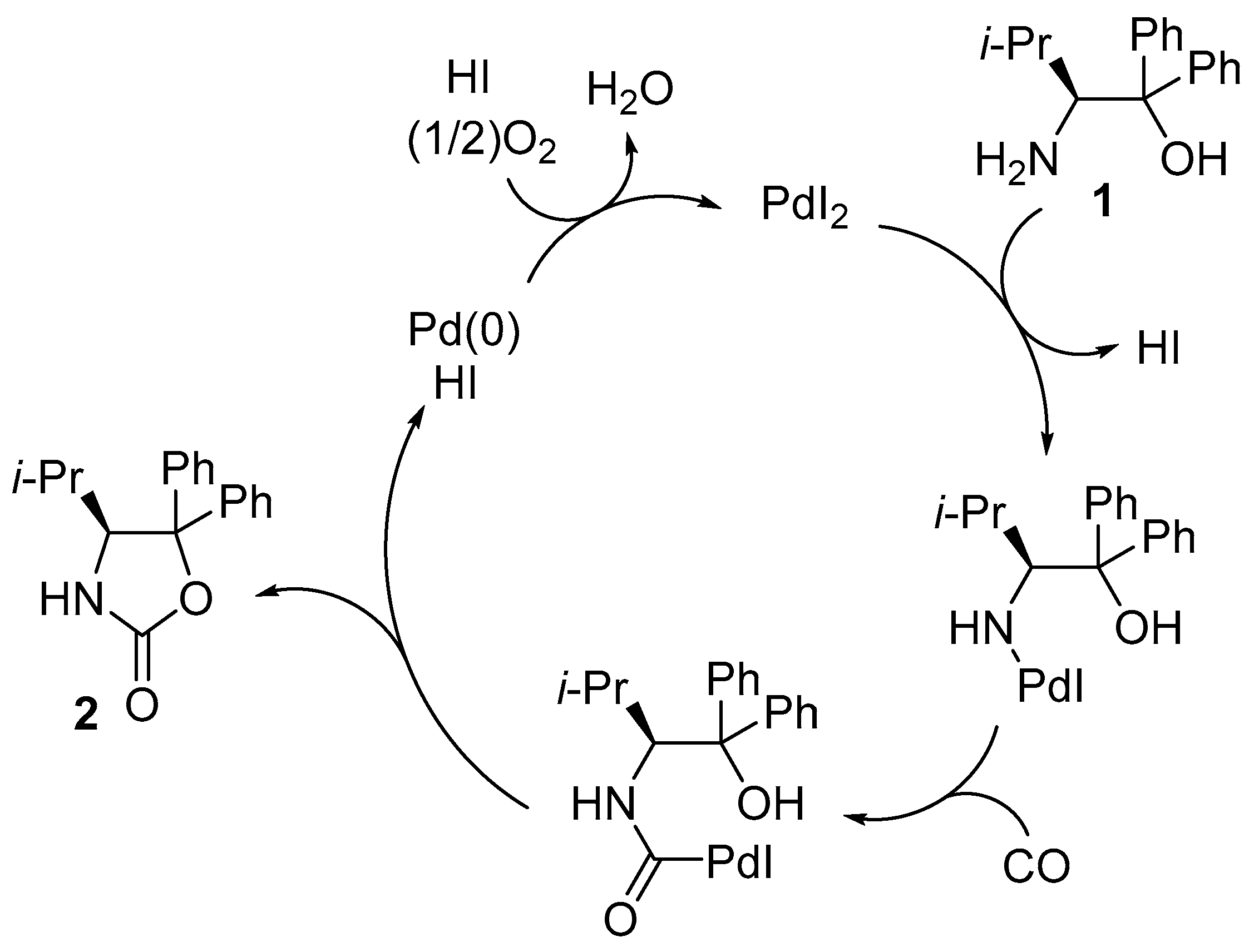
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Roger, C.; Roberts, J.A. Clinical Pharmacokinetics and Pharmacodynamics of Oxazolidinones. Clin. Pharmacokinet. 2018, 57, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Zadsirjan, V.; Heravi, M.M. Oxazolidinones as Chiral Auxiliaries in the Asymmetric 1,4-Conjugate Addition Reaction Applied to the Total Synthesis of Natural Products: A Supplemental Mini-Review. Curr. Org. Synth. 2018, 15, 3–20. [Google Scholar] [CrossRef]
- Gabriele, B. Synthesis of Heterocycles by Palladium-Catalyzed Carbonylative Reactions. In Advances in Transition-Metal Mediated Heterocyclic Synthesis; Solé, D., Fernández, I., Eds.; Academic Press-Elsevier: London, UK, 2018; Volume 3, pp. 55–127. ISBN 978-0-12-811651-7. [Google Scholar]
- Gabriele, B.; Mancuso, R.; Salerno, G. Oxidative Carbonylation as a Powerful Tool for the Direct Synthesis of Carbonylated Heterocycles. Eur. J. Org. Chem. 2012, 2012, 6825–6839. [Google Scholar] [CrossRef]
- Mancuso, R.; Raut, D.S.; Della Ca’, N.; Fini, F.; Carfagna, C.; Gabriele, B. Catalytic Oxidative Carbonylation of Amino Moieties to Ureas, Oxamides, 2-Oxazolidinones, and Benzoxazolones. ChemSusChem 2016, 8, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Mancuso, R.; Salerno, G.; Costa, M. An Improved Procedure for the Palladium-Catalyzed Oxidative Carbonylation of β-Amino Alcohols to Oxazolidin-2-ones. J. Org. Chem. 2003, 68, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Hintermann, T.; Seebach, D. A Useful Modification of the Evans Auxiliary: 4-Isopropyl-5,5-diphenyloxazolidin-2-one. Helv. Chim. Acta 1998, 81, 2093–2126. [Google Scholar] [CrossRef]
- Gibson, C.L.; Gillon, K.; Cook, S. A Study of 4-Substituted 5,5-Diaryl Oxazolidin-2-ones as Efficacious Chiral Auxiliaries. Tetrahedron Lett. 1998, 39, 6733–6736. [Google Scholar] [CrossRef]
- Gawley, R.E.; Zhang, P. 1-Magnesiotetrahydroisoquinolyloxazolines as Chiral Nucleophiles in Stereoselective Additions to Aldehydes: Auxiliary Optimization, Asymmetric Synthesis of (+)-Corlumine, (+)-Bicuculline, (+)-Egenine, and (+)-Corytensine, and Preliminary 13C NMR Studies of 1-Lithio- and 1-Magnesiotetrahydroisoquinolyloxazolines. J. Org. Chem. 1996, 61, 8103–8112. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.; Cook, S.; Gibson, C.L.; Kennedy, A.R. Highly Effective and Recyclable Chiral Auxiliaries: A Study of the Synthesis and Use of Three 4-Isopropyl-5,5-diaryloxazolidin-2-ones. J. Chem. Soc., Perkin Trans. 1 2001, 2001, 1538–1549. [Google Scholar] [CrossRef]
- O’Hagan, D.; Tavasli, M. A Short Synthesis of (S)-α-(Diphenylmethyl)alkyl Amines from Amino Acids. Tetrahedron: Asymm. 1999, 10, 1189–1192. [Google Scholar] [CrossRef]
- Yu, L.-T.; Ho, M.-T.; Chang, C.-Y.; Yang, T.-K. Asymmetric Zinc-Reformatsky Reaction of Evans Chiral Imide with Acetophenones and Its Application to the Stereoselective Synthesis of Triazole Antifungal Agents. Tetrahedron Asymm. 2007, 18, 949–962. [Google Scholar] [CrossRef]
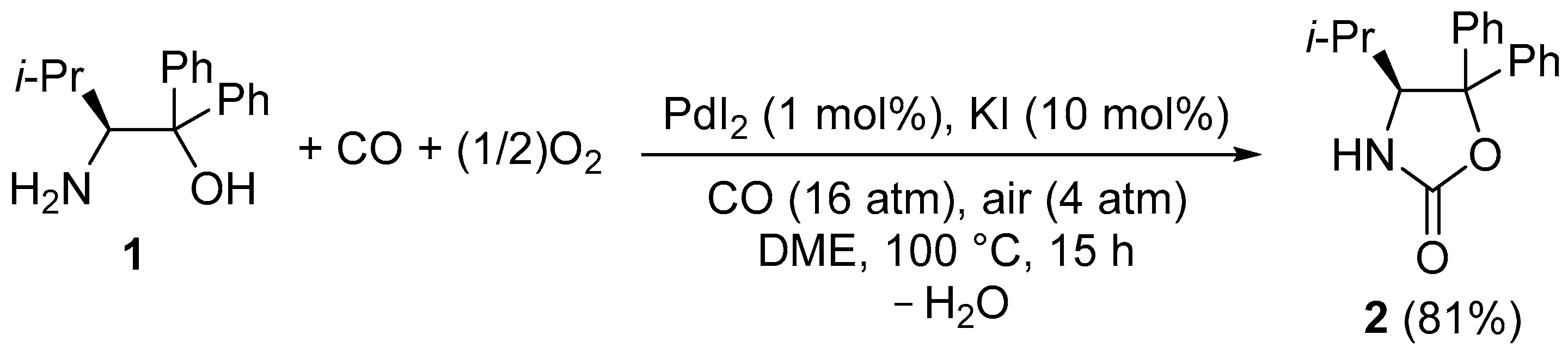
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, R.; Miliè, R.; Ziccarelli, I.; Novello, M.; Della Ca’, N.; Gabriele, B. (S)-4-Isopropyl-5,5-diphenyloxazolidin-2-one. Molbank 2018, 2018, M1017. https://doi.org/10.3390/M1017
Mancuso R, Miliè R, Ziccarelli I, Novello M, Della Ca’ N, Gabriele B. (S)-4-Isopropyl-5,5-diphenyloxazolidin-2-one. Molbank. 2018; 2018(3):M1017. https://doi.org/10.3390/M1017
Chicago/Turabian StyleMancuso, Raffaella, Rossana Miliè, Ida Ziccarelli, Mariangela Novello, Nicola Della Ca’, and Bartolo Gabriele. 2018. "(S)-4-Isopropyl-5,5-diphenyloxazolidin-2-one" Molbank 2018, no. 3: M1017. https://doi.org/10.3390/M1017
APA StyleMancuso, R., Miliè, R., Ziccarelli, I., Novello, M., Della Ca’, N., & Gabriele, B. (2018). (S)-4-Isopropyl-5,5-diphenyloxazolidin-2-one. Molbank, 2018(3), M1017. https://doi.org/10.3390/M1017








