[(η5-pentamethylcyclopentadienyl)(3-fluoro-N-methylbenzylamine-к1,N)dichlorido]iridium(III)
Abstract
1. Introduction
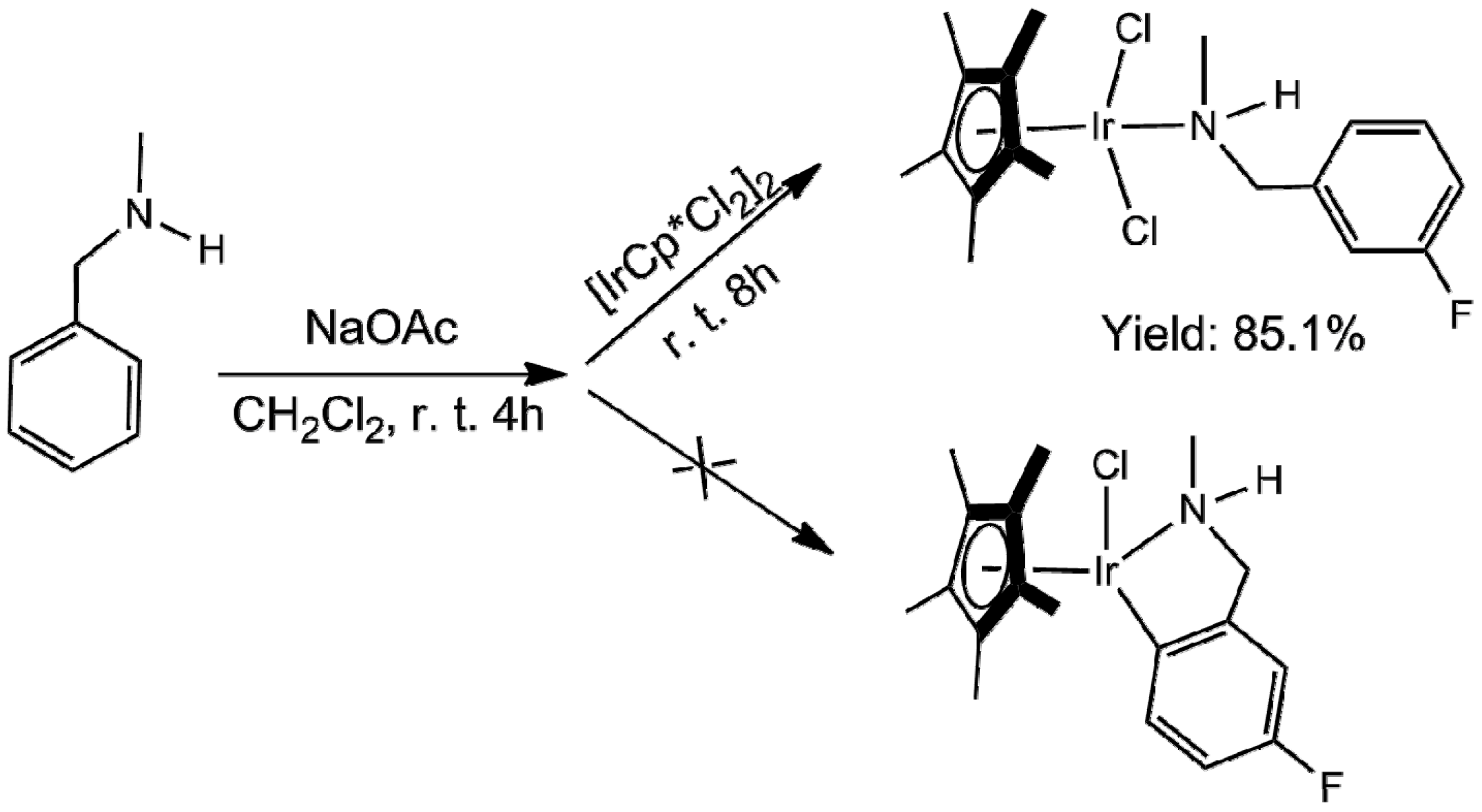
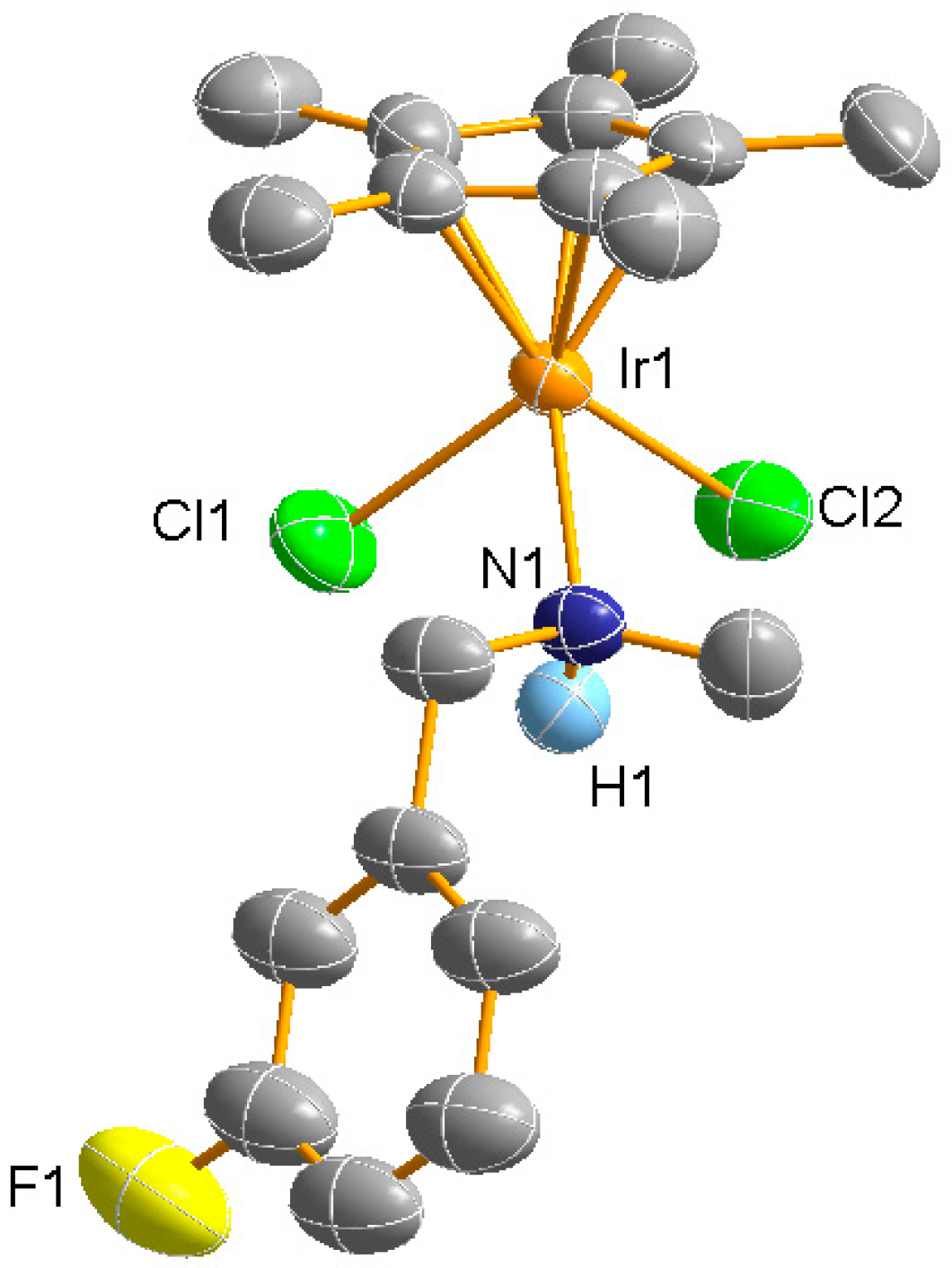
2. Results and Discussion
3. Materials and Methods
3.1. General Methods and Physical Measurements
3.2. Synthesis of [(η5-Cp*)Ir(C6H4FCH2NHCH3)Cl2]
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jerphagnon, T.; Gayet, A.J.A.; Berthiol, F.; Ritleng, V.; Mršić, N.; Meetsma, A.; Pfeffer, M.; Minnaard, A.J.; Feringa, B.L.; de Vries, J.G. Fast racemisation of chiral amines and alcohols by using cationic half-sandwich ruthena and iridacycle catalysts. Chem.-Eur. J. 2009, 15, 12780–12790. [Google Scholar] [CrossRef] [PubMed]
- Arita, S.; Koike, T.; Kayaki, Y.; Ikariya, T. Aerobic oxidation of alcohols with bifunctional transition-metal catalysts bearing C-N chelate ligands. Chem. Asian J. 2008, 3, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Barloy, L.; Issenhuth, J.T.; Weaver, M.G.; Pannetier, N.; Sirlin, C.; Pfeffer, M. Reaction of Chiral Secondary Amines with [(η5-C5Me5)MCl2]2 (M = Rh(III), Ir(III)): Cyclometalation with or without Dehydrogenation. Organometallics 2011, 30, 1168–1174. [Google Scholar] [CrossRef]
- Sortais, J.B.; Pannetier, N.; Holuigue, A.; Barloy, L.; Sirlin, C.; Pfeffer, M.; Kyritsakas, N. Cyclometalation of Primary Benzyl Amines by Ruthenium(II), Rhodium(III), and Iridium(III) Complexes. Organometallics 2007, 26, 1856–1867. [Google Scholar] [CrossRef]
- Haak, R.M.; Berthiol, F.; Jerphagnon, T.; Gayet, A.J.A.; Tarabiono, C.; Postema, C.P.; Ritleng, V.; Pfeffer, M.; Janssen, D.B.; Minnaard, A.J.; et al. Dynamic Kinetic Resolution of Racemic β-Haloalcohols: Direct Access to Enantioenriched Epoxides. J. Am. Chem. Soc. 2008, 130, 13508–13509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, L.; Tian, Z.; Tian, M.; Zhang, S.; Xu, Z.; Gong, P.; Zheng, X.; Zhao, J.; Liu, Z. Significant Effects of Counteranions on the Anticancer Activity of Iridium(III) Complexes. Chem. Commun. 2018, 54, 4421–4424. [Google Scholar] [CrossRef] [PubMed]
- He, X.D.; Tian, M.; Liu, X.C.; Tang, Y.; Shao, C.F.; Gong, P.W.; Liu, J.F.; Zhang, S.M.; Guo, L.H.; Liu, Z. Triphenylamine-appended half-sandwich Iridium(III) complexes and their biological applications. Chem.-Asian J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Li, J.J.; Zhang, S.M.; Guo, L.H.; He, X.D.; Kong, D.L.; Zhang, H.R.; Liu, Z. Half-sandwich Ruthenium(II) Complexes Containing N^N-chelated Imino-pyridyl Ligands That Are Selectively Toxic to Cancer Cells. Chem. Commun. 2017, 53, 12810–12813. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Guo, L.H.; Tian, Z.Z.; Tian, M.; Zhang, S.M.; Xu, K.; Qian, Y.C.; Liu, Z. Novel half-sandwich Iridium(III) imino-pyridyl complexes showing remarkable in vitro anticancer activity. Dalton Trans. 2017, 46, 15520–15534. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Romero-Canelón, I.; Qamar, B.; Hearn, J.M.; Habtemariam, A.; Barry, N.P.; Pizarro, A.M.; Clarkson, G.J.; Sadler, P.J. The Potent Oxidant Anticancer Activity of Organoiridium Catalysts. Angew. Chem. Int. Ed. 2014, 53, 3941–3946. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Romero-Canelón, I.; Habtemariam, A.; Clarkson, G.J.; Sadler, P.J. Potent Half-Sandwich Iridium(III) Anticancer Complexes Containing C∧N-Chelated and Pyridine Ligands. Organometallics 2014, 33, 5324–5333. [Google Scholar] [CrossRef] [PubMed]
- Soledad, B.L.; Abraha, H.; Clarkson, G.J.; Sadler, P.J. Organometallic cis-Dichlorido Ruthenium(II) Ammine Complexes. Eur. J. Inorg. Chem. 2011, 2011, 3257–3264. [Google Scholar]
- Cope, A.C.; Siekman, R.W. Formation of Covalent Bonds from Platinum or Palladium to Carbon by Direct Substitution. J. Am. Chem. Soc. 1965, 87, 3272–3273. [Google Scholar] [CrossRef]
- Cope, A.C.; Friedrich, E.C. Electrophilic aromatttic substitution reactions by platinum(II) and palladium(II) chlorides on N,N-dimethylbenzylamines. J. Am. Chem. Soc. 1968, 90, 909–913. [Google Scholar] [CrossRef]
- Han, Y.F.; Jin, G.X. Cyclometalated [Cp*M(C^X)] (M = Ir, Rh; X = N, C, O, P) complexes. Chem. Soc. Rev. 2014, 43, 2799–2823. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Mcardle, P. Oscail, a program package for small-molecule single-crystal crystallography with crystal morphology prediction and molecular modelling. J. Appl. Crystallogr. 2017, 50, 320–326. [Google Scholar] [CrossRef]
- Wang, C.L.; Liu, J.F.; Tian, Z.Z.; Tian, M.; Tian, L.J.; Zhao, W.; Liu, Z. Half-sandwich iridium N-heterocyclic carbene anticancer complexes. Dalton Trans. 2017, 46, 6870–6883. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Guo, L.; Zhang, S.; Liu, X.; Liu, Z. [(η5-pentamethylcyclopentadienyl)(3-fluoro-N-methylbenzylamine-к1,N)dichlorido]iridium(III). Molbank 2018, 2018, M999. https://doi.org/10.3390/M999
Kong D, Guo L, Zhang S, Liu X, Liu Z. [(η5-pentamethylcyclopentadienyl)(3-fluoro-N-methylbenzylamine-к1,N)dichlorido]iridium(III). Molbank. 2018; 2018(2):M999. https://doi.org/10.3390/M999
Chicago/Turabian StyleKong, Deliang, Lihua Guo, Shumiao Zhang, Xicheng Liu, and Zhe Liu. 2018. "[(η5-pentamethylcyclopentadienyl)(3-fluoro-N-methylbenzylamine-к1,N)dichlorido]iridium(III)" Molbank 2018, no. 2: M999. https://doi.org/10.3390/M999
APA StyleKong, D., Guo, L., Zhang, S., Liu, X., & Liu, Z. (2018). [(η5-pentamethylcyclopentadienyl)(3-fluoro-N-methylbenzylamine-к1,N)dichlorido]iridium(III). Molbank, 2018(2), M999. https://doi.org/10.3390/M999




