Abstract
The two precursors 3-chloro-2-methylphenylthiourea 2 and 4-fluorophenacyl bromide 4 are reacted under Hantzsch thiazole synthesis condition to yield the new compound N-(3-chloro-2-methylphenyl)-4-(4-fluorophenyl)-1,3-thiazol-2-amine 5. The IR, 1H-NMR, 13C-NMR and mass spectral data are presented, along with its in vitro antibacterial activity against Staphylococcus aureus and Chromobacterium violaceum.
1. Introduction
Hantzsch synthesis is always a preferred reaction for synthesizing thiazole and its derivatives. It is a facile reaction between a substituted thiourea with α-halo ketones in presence of a green solvent ethanol. The thiazole chemistry has flourished, bringing with it the advent of many drugs derived from it, such as Sulfathiazole, an antimicrobial, Abafungin, an antifungal, antiretrovirals (Ritonavir) and antineoplastics (Bleomycin and Tiazofurin), to name a few [1]. In recent years, the use of thiazole scaffolds has remarkably improved efficacy of the drugs that are put into practice for the treatment of HIV infections [2], various types of cancer [3], hypertension [4], schizophrenia [5], allergies [6] and also bacterial and fungal infections. Nitazoxanide is a marketed antimicrobial drug thatis highly effective against H. pylori when administered with omeprazole for 7 days. It has demonstrated no cross resistance to metronidazole [7,8,9].
Darbufelone{(5Z)-2-amino-5-[(3,5-ditert-butyl-4-hydroxyphenyl)methylidene]-1,3-thiazol-4-one} is a marketed anti-inflammatory drug that has also proved its potential as a lung cancer cell growth inhibitor [10]. The presence of chlorine in low molecular weight compounds affects its biological activity by altering the electrophilicity of carbon in C-Cl bond. This facilitates its reaction with bionucleophiles such as DNA bases or a regulating protein through the displacement of chlorine. It acts as a starting point for mutations in cancer regulating targets; hence, this chemistry is effectively used for chemotherapy against cancer [11]. Studies on the biological activity of fluorine substitution have revealed that the C-F bond (van der Waals radius = 1.47 Å) has amore similar bond length tothat of the C-O bond (van der Waals radius = 1.52 Å) than to that ofthe C-H bond (van der Waals radius = 1.2 Å); hence, it can be considered as isosteric with oxygen. However, fluorine is still the smallest substituent that can be used as a replacement for the C-H bond, and is also more lipophilic than hydrogen, influencing the ADME properties of small molecule-based drugs. The similarity of the C-F bond to the C-O and C-H bonds enhances the effectiveness of many fluorinated analogues in binding with macromolecular recognition sites as a natural substrate [12].The addition of a methyl group as a substituent in the aryl ring is found to alter the biological activity considerably. It has been reported that a boost of a factor of 10 or more is found with an 8% frequency, and a 100-fold boost is a 1 in 200 event, based on the analysis of more than 2000 cases. It is worthwhile noting that when a methyl group is at the ortho position on an aryl ring, it is effectively bound to the target, influencing favorable conformational change. The gain in the activity arises due to the entombment of the methyl group in a hydrophobic region of the protein [13].
Hence, owing to the demand for the synthesis of new bioactive compounds derived from thiazole, the title compound 5 with chloro, fluoro and methyl substitution at the ortho position is designed and synthesized starting from 3-chloro-2-methylaniline via a two-step sequence reaction, and is subsequently characterized and evaluated for its antibacterial activity.
2. Results and Discussion
2.1. Chemistry
The synthesis of the new compound 5 is outlined in Scheme 1. The key intermediate 3-chloro-2-methylphenylthiourea 2 was obtained from the reaction of ammonium thiocyanate with 3-chloro-2-methylaniline 1 in concentrated HCl under reflux conditions [14]. 4-Fluorophenacylbromide 4 was prepared by bromination of 4-fluoroacetophenone 3 [15]. The title compound, N-(3-chloro-2-methylphenyl)-4-(4-fluorophenyl)-1,3-thiazol-2-amine 5 was synthesized by refluxing a mixture of 3-chloro-2-methylphenylthiourea 2 and 4-fluorophenacylbromide 4 in ethanol solvent in the absence of a catalyst.
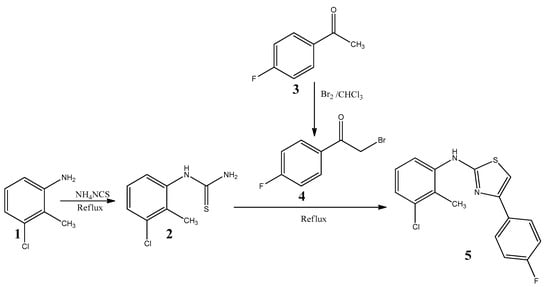
Scheme 1.
Synthesis of N-(3-chloro-2-methylphenyl)-4-(4-fluorophenyl)-1,3-thiazol-2-amine 5.
The formation of the title compound 5 was confirmed by its spectral data. The IR spectrum of compound 5 showed prominent band at 3130 cm−1 due to N-H stretching. Absorption bands seen at 3045 cm−1 and 2926, 2862 cm−1 were assigned to stretching of aromatic C-H and alkyl C-H bonds; an absorption band appeared at 1554 cm−1 assigned to -C=N stretching frequency. The prominent absorption bands appearing at 1056 cm−1 accounted for C-F stretching, and at 725 cm−1 for C-Cl stretching frequencies, confirming the formation of the compound. In the 1H-NMR spectrum of compound 5, the methyl (CH3) protons had resonated as a singlet at δ 2.55 ppm, accounting for three protons. The -NH proton appeared as a singlet at δ 6.59 ppm, accounting forone proton; whereas the eight remaining protons resonated in the aromatic region between δ 7.18 and δ 7.83 ppm. The signal appearing at δ 7.28 ppm could be attributed to the thiazole ring proton. A doublet seen at δ 7.18–7.20 ppm (J = 8 Hz) could be assigned to the ortho protons of the p-fluorophenyl ring, whereas meta protons had resonated at δ 7.79–7.83 ppm as a quintet (J = 5 Hz), accounting for two protons. The splitting in this signal could be due to the meta coupling with fluorine. The three protons of the chloro methyl-substituted phenyl ring appeared as two doublets δ 7.34–7.36 ppm (J = 8 Hz), accounting for one proton each; however, the meta proton resonated at δ 7.24–7.26 ppm (J = 8 Hz) as a doublet merged with the CDCl3 proton. In 13C-NMR (100 MHz, DMSO-d6 solvent), the signal for methyl carbon was seen at δ 14.9 ppm. The signals for carbon atoms of the p-fluorophenyl ring appeared as seven doublets due to long-range fluorine coupling. All other signals could be accounted for the number of remaining carbons present in the molecule. The formation of compound 5 was further confirmed by its mass spectrum, which showed a molecular ion peak at m/z 319.00 (M+ + 1) consistent with its molecular formula C16H12ClFN2S. An isotopic peak was also seen at m/z 321 (M+ + 3) in 3:1 ratio, which confirmed the presence of chlorine in the molecule. The elemental analysis gave satisfactory values for the percentage of C, H and N present in the molecule, and is presented in the experimental section.
2.2. Antibacterial Activity
The new compound 5 was screened for in vitro antibacterial activity against two bacterial strains: gram-positive Staphylococcus aureus and gram-nagative Chromobacterium violaceum. The results are expressed in terms of average zone of inhibition in mm for triplicates. These results were compared to those of the standard drug Streptomycin (Table 1).

Table 1.
Antibacterial activity of compound 5.
Compound 5 showed convincing antibacterial activity against two tested bacterial strains S. aureus and C. violaceum with 20.5 ± 0.4 mm and 17.0 ± 0.3 mm zones of inhibition, respectively, but the activity was lower than that of the standard drug Streptomycin, which possesses 36.6 ± 0.3 mm and 29.1 ± 0.2 mm, respectively. It was also noted that DMSO used as solvent had no effect on antibacterial activity.
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
All required reagents were used as received from suppliers without further purification. The melting point was determined in an open capillary tube, and is uncorrected. The IR-spectrum was recorded on Shimadzu FT-IR Prestige-21 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) in KBr pellets and is expressed in cm−1. The mass spectrum was obtained using Shimadzu LC MS-8030 mass spectrometer (Shimadzu Corporation, Kyoto, Japan) operating at 70 eV. The 1H-NMR and 13C-NMR spectra were recorded on a Bruker AVANCE II 400 MHz instrument (Bruker BioSpin AG, Fällanden, Switzerland) in CDCl3 /DMSO-d6 solvent and TMS as an internal standard. The purity of the compound and completion of the reaction were monitored by TLC (Merck KGaA, Darmstadt, Germany) using Merck silica gel 60 F 256 coated aluminum with petroleum ether:ethylacetate (8:2) as mobile phase. The elemental analysis was carried out using a CHNSO analyzer (Model: FLASH EA 1112 series make: Thermo finnigan, (Thermo Electron Corporation. Swagelok®, Milano, Italy).
3.1.2. Synthesis of N-(3-Chloro-2-methylphenyl)-4-(4-fluorophenyl) 1,3-thiazol-2-amine 5
Equimolar amounts of 3-chloro-2-methylphenylthiourea 2 (2 g, 0.01 mol) and 4-fluorophenacylbromide 4 (2.14 g, 0.01 mol) in 50 mL ethanol were heated under reflux for 24 h. After cooling, separated solid was filtered, dried and recrystallized from ethanol to yield compound 5. White solid, M.p.: 200–202 °C; Yield: 65%; IR (KBr, cm−1): 3130 (NH), 3045 (CH aromatic), 2926, 2862 cm−1 (CH aliphatic), 1554 (C=N) 1056 cm−1(C-F stretch) and 725 cm−1(C-Cl stretch). 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.55 (s, 3H, CH3); 6.59 (s, 1H, NH); 7.18–7.20 (d, J = 8 Hz, 2H, fluorophenyl H-3, H-5); 7.24–7.26 (d, J = 8 Hz, 1H, chloromethylphenyl H-5); 7.28 (s, 1H,thiazole H-5); 7.34–7.36 (d, J = 8 Hz, 1H, chloromethylphenyl H-6); 7.43–7.45 (d, J = 8 Hz, 1H, chloromethylphenyl H-4); 7.79–7.83 (q, 2H, fluorophenyl H-2, H-6). 13C-NMR (DMSO-d6, 100 MHz) δ ppm: 14.9 (CH3), 102.7, 120.4, 120.7, 124.5, 127.7, 127.7, 129.95, 130.0, 134.3, 134.4, 140.1, 140.3, 146.1, 164.0, 166.1. LC-MS (m/z): 319.00 (M+ + 1) for Mol formula: C16H12ClFN2S. Elemental Analysis, % Composition: C: 60.28 (Cald.), 60.30 (Found); H: 3.79 (Cald.), 3.76 (Found); N: 8.79 (Cald.), 8.90 (Found).
Copies of these spectra are provided in the Supplementary Materials Figure S1 to S4.
3.2. Antibacterial Activity
The disc diffusion method [16] was used for the antibacterial study against the Staphylococcus aureus (ATTC 2043) and Chromobacterium violaceum (ATTC 2216) bacterial isolates. Prepared Muller-Hinton Agar media was poured onto sterile petri plates and allowed to solidify. 20 µL of suspension containing approximately 5 × 105 colony-forming units (CFU)/mL of bacteria grown for 24 h in nutrient broth were inoculated on agar surface of each petri plate under aseptic conditions using a sterile cotton swab. Sterile paper discs were impregnated with 15 µL of test solution (compound 5 at 3.14 × 10−3 M in DMSO), then placed on inoculated agar plates at equal distance along with the control (DMSO) and standard drug (Streptomycin/20 µg/Disc). The plates were then incubated at 37 °C for 24 h. After incubation, zones of inhibition were measured in mm. Tests were carried out in triplicate and the results are expressed as mean ± SD.
Supplementary Materials
The following are available online: www.mdpi.com/1422-8599/2018/1/M975, Figure S1: 1H-NMR spectrum of the synthesized compound 5, Figure S2: 13C-NMR spectrum of the synthesized compound 5, Figure S3: IR spectrum of the synthesized compound 5, Figure S4: Mass spectrum of the synthesized compound 5.
Acknowledgments
The authors are thankful to the Directors, SAIF Punjab University, USIC Mangalore University for providing facility for spectral analysis. One of others (Nadine Uwabagira) is also thankful to Indian Council for Culture Relations (ICCR) for granting scholarship.
Author Contributions
All authors contributed equally to all activities: design, synthesis, biological study, results interpretation and manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siddiqui, N.; Arshad, M.F.; Ahsan, W.; Alam, M.S. Thiazoles: A valuable insight into the recent advances and biological activities. Int. J. Pharm. Sci. Drug Res. 2009, 1, 136–143. [Google Scholar]
- Madni, M.; Hameed, S.; Ahmed, M.N.; Tahir, M.N.; Al-Masoudi, N.A.; Pannecouque, C. Synthesis, crystal structure, anti-HIV and antiproliferative activity of new pyrazolylthiazole derivatives. Med. Chem. Res. 2017, 26, 2653–2665. [Google Scholar] [CrossRef]
- Sadek, B.; Al-Tabakha, M.M.; Fahelelbom, K.M.S. Antimicrobial prospect of newly synthesized 1,3-thiazole derivatives. Molecules 2011, 16, 9386–9396. [Google Scholar] [CrossRef] [PubMed]
- Patt, W.C.; Hamilton, H.W.; Taylor, M.D.; Ryan, M.J.; Taylor, D.G., Jr.; Connolly, C.J.C.; Doherty, A.M.; Klutchko, S.R.; Sircar, I. Structure-activity relationships of a series of 2-amino-4-thiazole-containing renin inhibitors. J. Med. Chem. 1992, 35, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Jean, J.C.; Wise, L.D.; Caprathe, B.W.; Tecle, H.; Bergmeier, S.; Humblet, C.C.; Heffner, T.G.; Meltzner, L.T.; Pugsley, T.A. 4-(1,2,5,6-Tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: A novel class of compounds with central dopamine agonist properties. J. Med. Chem. 1990, 33, 311–317. [Google Scholar] [CrossRef]
- Hargrave, K.D.; Hess, F.K.; Oliver, J.T. N-(4-substituted-thiazolyl)oxamic acid derivatives, a new series of potent, orally active antiallergy agents. J. Med. Chem. 1983, 26, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Narayana, B.; Vijaya Raj, K.K.; Ashalatha, B.V.; Suchetha Kumari, N. Antibacterial and antifungal studiesn some new acetylcinnolines and cinnolinylthiazole derivatives. Ind. J. Chem. Sect. B 2006, 45B, 1704–1709. [Google Scholar]
- Sarojini, B.K.; Krishna, B.G.; Darshanraj, C.G.; Bharath, B.R.; Manjunatha, H. Synthesis, characterization, in vitro and molecular docking studies of new 2,5-dichloro thienyl substituted thiazole derivatives for antimicrobial properties. Eur. J. Med. Chem. 2010, 45, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Me’graud, F.; Occhialini, A.; Rossignol, J.F. Nitazoxanide, a potential drug for eradication of Helicobacter pylori with no cross-resistance to metronidazole. Antimicrob. Agents Chemother. 1998, 42, 2836–2840. [Google Scholar]
- Ye, X.; Zhou, W.; Li, Y.; Sun, Y.; Zhang, Y.; Ji, H.; Lai, Y. Darbufelone, a novel anti-inflammatory drug, induces growth inhibition of lung cancer cells both in vitro and in vivo. Cancer Chemother. Pharmacol. 2010, 66, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Naumann, K. Influence of chlorine substituents on biological activity of chemicals. J. Prakt. Chem. 1999, 341, 417–435. [Google Scholar] [CrossRef]
- Kirk, K.L. Fluorine in medicinal chemistry: Recent therapeutic applications of fluorinated small molecules. J. Fluor. Chem. 2006, 127, 1013–1029. [Google Scholar] [CrossRef]
- Leung, C.S.; Leung, S.S.F.; Tirado-Rives, J.; Jorgensen, W.L. Methyl effects on protein–ligand binding. J. Med. Chem. 2012, 55, 4489–4500. [Google Scholar] [CrossRef] [PubMed]
- Yavari, I.; Hossaini, Z.; Sabbaghan, M.; Ghazanfarpour-Darjani, M. A one-pot synthesis of functionalized thiazoles from acid chlorides, secondary amines, ethyl bromopyruvate, and ammonium thiocyanate. Mol. Divers. 2009, 13, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Malini, K.V.; Rao, B.S.; Sarojini, B.K.; Kumari, N.S. Synthesis of some new 2,4-disubstituted thiazoles as possible antibacterial and anti-inflammatory agents. Eur. J. Med. Chem. 2003, 38, 313–318. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).