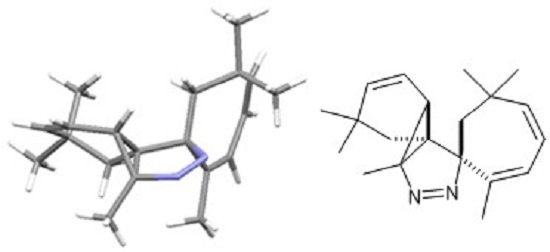
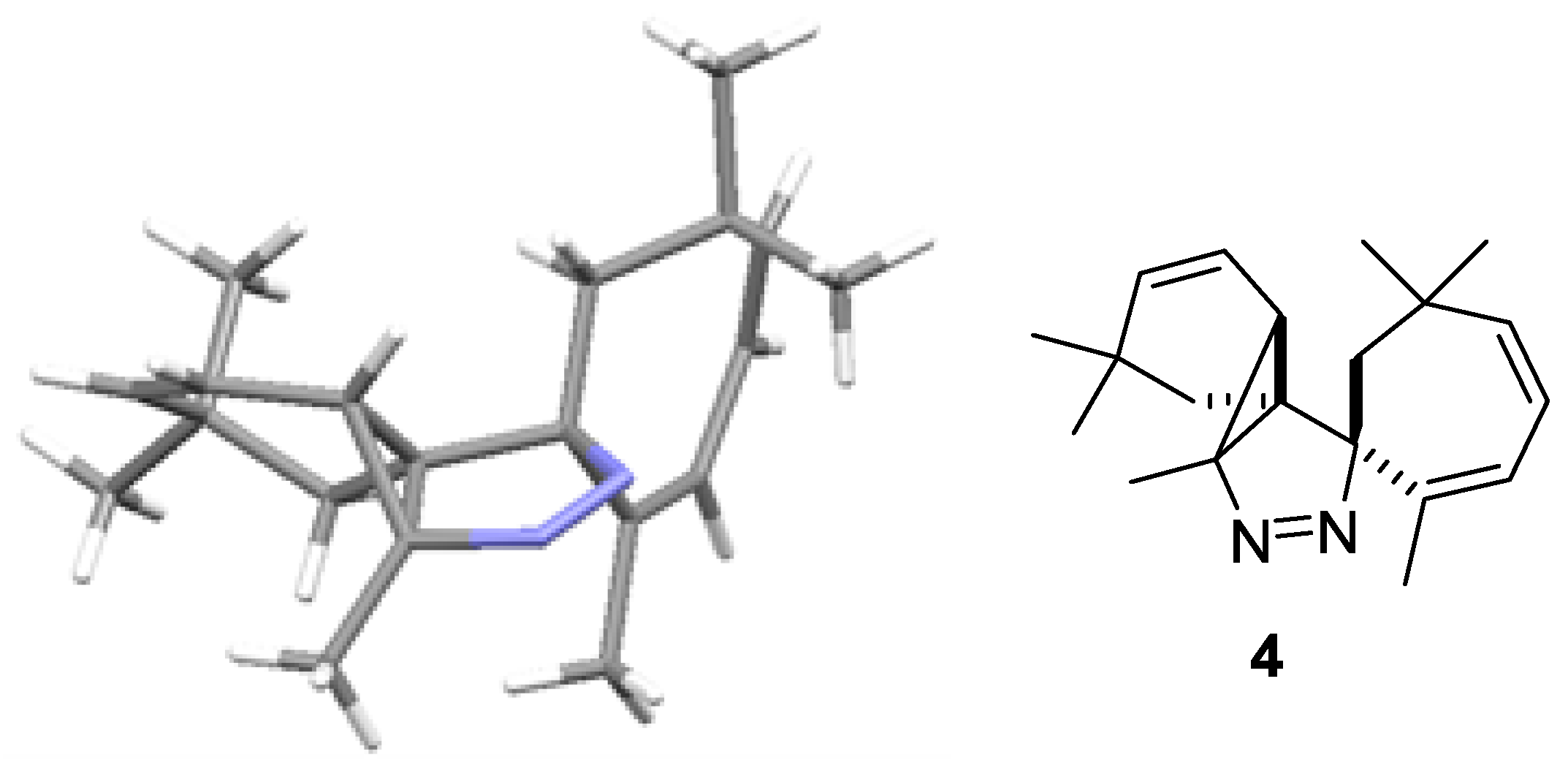
Rac-2′,3a,6,6,6′,6′-Hexamethyl-3a,3b,6,7-tetra-hydrospiro-[benzo[2,3]cyclopropa[1,2-c]pyrazole-1,1′-cyclo-hepta[2,4]diene]
Abstract
:1. Introduction
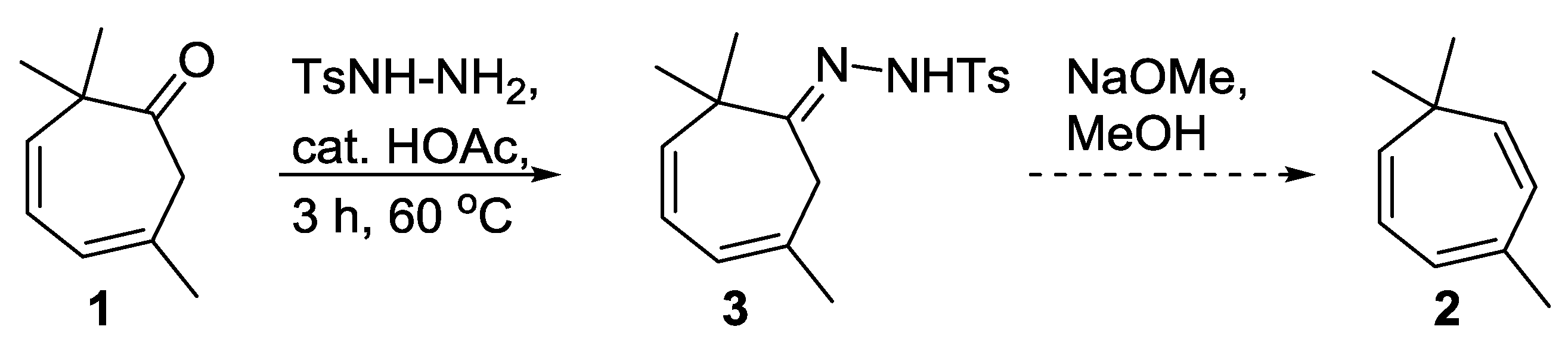
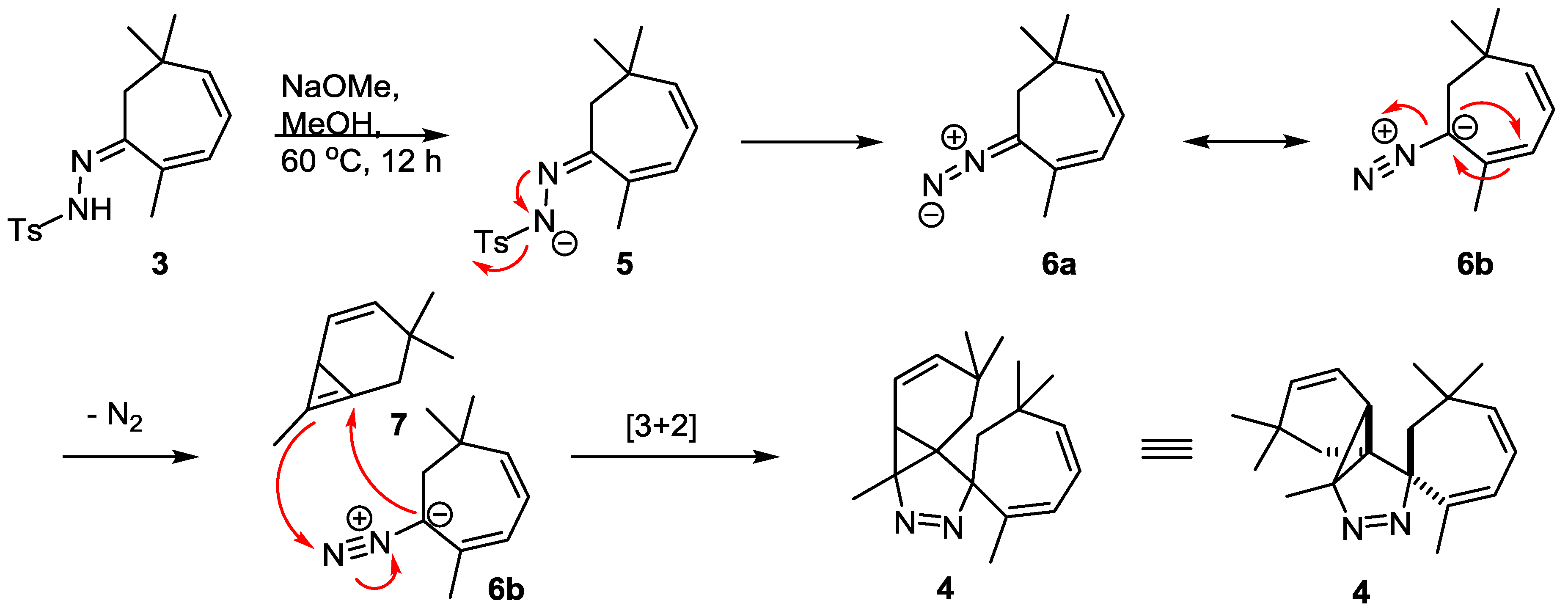
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Rojas, C.M. Molecular Rearrangements in Organic Synthesis, 1st ed.; John Wiley & Sons: New York, NY, USA, 2015; pp. 1–750. [Google Scholar]
- Yadav, V.K.; Babu, K.G. A remarkably efficient Markovnikov hydrochlorination of olefins and transformation of nitriles into imidates by use of AcCl and an alcohol. Eur. J. Org. Chem. 2005, 452–456. [Google Scholar] [CrossRef]
- Selezneva, N.K.; Gimalova, F.A.; Valeev, R.F.; Miftakhov, M.S. Carvone hydrochloride in the synthesis of thiazole-containing C11-C21-building block of epithilones gem-dimethylcyclopropane analogs. Russ. J. Org. Chem. 2010, 46, 191–197. [Google Scholar] [CrossRef]
- Bamford, W.R.; Stevens, T.S. The decomposition of p-tolylsulfonylhydrazones by alkali. J. Chem. Soc. 1952, 4735–4740. [Google Scholar] [CrossRef]
- Adlington, R.M.; Barrett, A.G.M. Recent applications of the Shapiro reaction. Acc. Chem. Res. 1983, 16, 55–59. [Google Scholar] [CrossRef]
- Romanenko, E.P.; Tkachev, A.V. Identification by GC-MS of cymene isomers 3,7,7-trimethylcyclohepta-1,3,5-heptatriene in essential oils. Chem. Nat. Compd. 2006, 42, 699–701. [Google Scholar] [CrossRef]
- CCDC 1562021 contains the X-ray structure crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk)
- Deng, Y.; Jing, C.; Doyle, M.P. Dinitrogen extrusion from enoldiazo compounds under thermal conditions: synthesis of donor-acceptor cyclopropenes. Chem. Commun. 2015, 51, 12924–12927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kubicki, J.; Platz, M.S. Ultrafast UV-visible and infrared spectroscopic observation of a singlet vinylcarbene and intramolecular cyclopropenation reaction. J. Am. Chem. Soc. 2009, 131, 13602–13603. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jing, C.; Zavalij, P.; Doyle, M.P. Hg(OTf)2 catalysed intramolecular 1,4-addition of donor-acceptor cyclopropenes to arenes. Org. Lett. 2015, 17, 4312–4315. [Google Scholar] [CrossRef] [PubMed]
- Ertelt, M.; Hrovat, D.A.; Thatcher Borden, W.; Sander, W. Heavy-atom tunneling in the ring opening of a strained cyclopropene at very low temperatures. Chem. Eur. J. 2014, 20, 4713–4720. [Google Scholar] [CrossRef] [PubMed]
- Instead of a cyclopropene species (7) it is also conceivable that a carbene is formed upon loss of nitrogen gas that subsequently is involved in the dipolar cycloaddition.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumann, M.; Lapraille, S.; Baxendale, I.R. Rac-2′,3a,6,6,6′,6′-Hexamethyl-3a,3b,6,7-tetra-hydrospiro-[benzo[2,3]cyclopropa[1,2-c]pyrazole-1,1′-cyclo-hepta[2,4]diene]. Molbank 2017, 2017, M948. https://doi.org/10.3390/M948
Baumann M, Lapraille S, Baxendale IR. Rac-2′,3a,6,6,6′,6′-Hexamethyl-3a,3b,6,7-tetra-hydrospiro-[benzo[2,3]cyclopropa[1,2-c]pyrazole-1,1′-cyclo-hepta[2,4]diene]. Molbank. 2017; 2017(3):M948. https://doi.org/10.3390/M948
Chicago/Turabian StyleBaumann, Marcus, Sophie Lapraille, and Ian R. Baxendale. 2017. "Rac-2′,3a,6,6,6′,6′-Hexamethyl-3a,3b,6,7-tetra-hydrospiro-[benzo[2,3]cyclopropa[1,2-c]pyrazole-1,1′-cyclo-hepta[2,4]diene]" Molbank 2017, no. 3: M948. https://doi.org/10.3390/M948
APA StyleBaumann, M., Lapraille, S., & Baxendale, I. R. (2017). Rac-2′,3a,6,6,6′,6′-Hexamethyl-3a,3b,6,7-tetra-hydrospiro-[benzo[2,3]cyclopropa[1,2-c]pyrazole-1,1′-cyclo-hepta[2,4]diene]. Molbank, 2017(3), M948. https://doi.org/10.3390/M948