2-[(2,6-Dimethylmorpholin-4-yl)methyl]-4-[(E)-2-{3-[(E)-2-{3-[(2,6-dimethylmorpholin-4-yl)methyl]-4-hydroxy-5-methoxyphenyl}ethenyl]-1H-pyrazol-5-yl}ethenyl]-6-methoxyphenol
Abstract
:1. Introduction
2. Results and Discussion
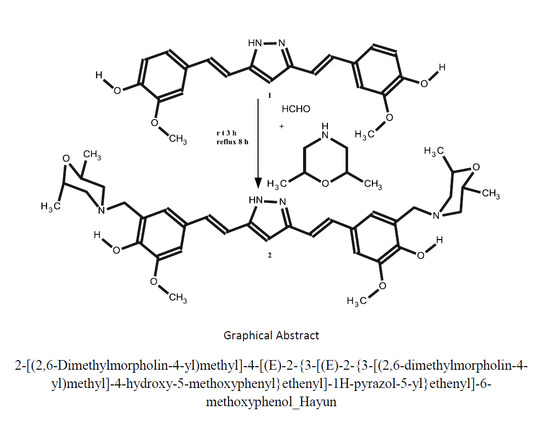
2.1. Chemistry
2.2. Solubility Evaluation
3. Materials and Methods
3.1. General
3.2. Synthesis of 2-[(2,6-Dimethylmorpholin-4-yl)methyl]-4-[(E)-2-{3-[(E)-2-{3-[(2,6-dimethylmorpholin-4-yl) methyl]-4-hydroxy-5-methoxyphenyl}ethenyl]-1H-pyrazol-5-yl}ethenyl]-6-methoxyphenol (2)
3.3. Solubility Evaluation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahsan, M.J.; Khalilullah, H.; Yasmin, S.; Jadav, S.S.; Govindasamy, J. Synthesis, characterisation, and in vitro anticancer activity of curcumin analogues bearing pyrazole/pyrimidine ring targeting EGFR Tyrosine Kinase. BioMed Res. Int. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Narlawar, R.; Pickhardt, M.; Leuchtenberger, S.; Baumann, K.; Krause, S.; Dyrks, T.; Weggen, S.; Mandelkow, E.; Schmidt, B. Curcumin-derived pyrazoles and isoxazoles: Swiss army knives or blunt tools for Alzheimer’s disease. ChemMedChem. 2008, 3, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Karmodiya, K.; Surolia, N.; Surolia, A. Synthesis and exploration of novel curcumin analogues as antimalarial agents. Bioorg. Med. Chem. 2008, 16, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Selvam, C.; Jachak, S.M.; Thilagavathi, R.; Chakraborti, A.K. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2005, 15, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.Y.; Bhosale, R.B.; Shirame, S.P.; Patil, S.B.; Kulkarni, S.D. PEG mediated synthesis and biological evaluation of asymmetrical pyrazole curcumin analogues as potential analgesic, anti-inflammatory and antioxidant agents. Chem. Biol. Drug Des. 2015, 85, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tang, H.-J.; Xu, J.J.; Ye, J.; Tian, H.-Y.; Li, Y.-L.; Jiang, R.-W. Rapid synthesis of curcuminoid pyrazoles with antiviral effects through one-pot combinatorial modification of total curcuminoids. Res. Rep. Med. Chem. 2015, 5, 41–47. [Google Scholar]
- Shioorkar, M.G.; Ubale, M.B. Simple and efficient microwave assisted PEG mediated synthesis of pyrazole analogues of curcumin. Der Pharma Chem. 2015, 7, 274–277. [Google Scholar]
- Nieto, C.I.; Claramunt, R.M.; Cabildo, M.P.; Cornago, M.P.; Sanz, D.; García, J.A.; Torralba, M.C.; Torres, M.R.; Elguero, J.; López, A.; et al. Fluorination effects on NOS inhibitory activity of pyrazoles related to curcumin. Molecules 2015, 20, 15643–15665. [Google Scholar] [CrossRef] [PubMed]
- Claramunt, R.M.; Nieto, C.I.; Sanz, D.; Elguero, J. Curcumin derived pyrazoles and related compounds. Afinidad LXXIV 2016, 576, 259–268. [Google Scholar]
- MarvinSketch 17.17.0. Chemaxon Ltd., 1998–2017. Available online: http://www.chemaxon.com (accessed on 2 August 2017).
- Reddy, M.V.B.; Su, C.R.; Chiou, W.F.; Liu, Y.N.; Chen, R.Y.; Bastow, K.F.; Lee, K.H.; Wu, T.S. Design, synthesis, and biological evaluation of Mannich bases of heterocyclic chalcone analogs as cytotoxic agents. Bioorg. Med. Chem. 2008, 16, 7358–7370. [Google Scholar] [CrossRef] [PubMed]
- Subramaniapillai, S.G. Mannich reaction: A versatile and convenient approach to bioactive skeletons. J. Chem. Sci. 2013, 125, 467–482. [Google Scholar] [CrossRef]
- Roman, G. Mannich bases in medicinal chemistry and drug design. Eur. J. Med. Chem. 2015, 89, 743–816. [Google Scholar] [CrossRef] [PubMed]
- Geschickter, C.F.; Meadow, J.R. Curcumin Derivatives. U.S. Patent Office 3, 479,345, 18 November 1969. [Google Scholar]
- Yerdelen, K.O.; Gul, H.I.; Sakagami, H.; Umemura, N. Synthesis and biological evaluation of 1,5-bis(4-hydroxy-3methoxyphenyl)penta-1,4-dien-3-one and its aminomethyl derivatives. J. Enzyme Inhib. Med. Chem. 2014, 30, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Available online: http://www.sigmaaldrich.com/spectra/fnmr/FNMR000707.PDF (accessed on 21 July 2017).
- Xu, G.; Yang, X.; Lei, P.; Liu, X.; Zhang, X.; Ling, Y. Synthesis ang fungicidal activity study of novel daphneolone analogs with 2,6-dimethylmorpholine. Chin. Chem. Lett. 2016, 27, 555–558. [Google Scholar] [CrossRef]
- Knittel, J.J.; Zavod, R.M. Drug Design and Relationship of Functional Groups to Pharmacologic Activity. In Foye’s Principles of Medicinal Chemistry, 7th ed.; Lemke, T.L., Williams, D.A., Roche, V.F., Zito, S.W., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 29–60. [Google Scholar]
- Dolai, S.; Shi, W.; Corbo, C.; Sun, C.; Averick, S.; Obeysekera, D.; Farid, M.; Alonso, A.; Banerjee, P.; Raja, K. “Clicked” sugar-curcumin conjugate: Modulator of amyloid-β and Tau peptide aggregation at ultralow concentrations. ACS Chem. Neurosci. 2011, 2, 694–699. [Google Scholar] [CrossRef] [PubMed]


| Compound | Molecular Formula | Molecular Weight | λ Max | Solubility 1 (mg/L) |
|---|---|---|---|---|
| Curcumin | C21H20O6 | 368.12 | 428.0 | 3.2 |
| 1 | C21H20N2O4 | 364.14 | 333.5 | 3.1 |
| 2 | C35H46N4O6 | 618.35 | 333.0 | 9.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Untung, J.; Iskandarsyah, I.; Hayun, H. 2-[(2,6-Dimethylmorpholin-4-yl)methyl]-4-[(E)-2-{3-[(E)-2-{3-[(2,6-dimethylmorpholin-4-yl)methyl]-4-hydroxy-5-methoxyphenyl}ethenyl]-1H-pyrazol-5-yl}ethenyl]-6-methoxyphenol. Molbank 2017, 2017, M949. https://doi.org/10.3390/M949
Untung J, Iskandarsyah I, Hayun H. 2-[(2,6-Dimethylmorpholin-4-yl)methyl]-4-[(E)-2-{3-[(E)-2-{3-[(2,6-dimethylmorpholin-4-yl)methyl]-4-hydroxy-5-methoxyphenyl}ethenyl]-1H-pyrazol-5-yl}ethenyl]-6-methoxyphenol. Molbank. 2017; 2017(3):M949. https://doi.org/10.3390/M949
Chicago/Turabian StyleUntung, Joko, Iskandarsyah Iskandarsyah, and Hayun Hayun. 2017. "2-[(2,6-Dimethylmorpholin-4-yl)methyl]-4-[(E)-2-{3-[(E)-2-{3-[(2,6-dimethylmorpholin-4-yl)methyl]-4-hydroxy-5-methoxyphenyl}ethenyl]-1H-pyrazol-5-yl}ethenyl]-6-methoxyphenol" Molbank 2017, no. 3: M949. https://doi.org/10.3390/M949
APA StyleUntung, J., Iskandarsyah, I., & Hayun, H. (2017). 2-[(2,6-Dimethylmorpholin-4-yl)methyl]-4-[(E)-2-{3-[(E)-2-{3-[(2,6-dimethylmorpholin-4-yl)methyl]-4-hydroxy-5-methoxyphenyl}ethenyl]-1H-pyrazol-5-yl}ethenyl]-6-methoxyphenol. Molbank, 2017(3), M949. https://doi.org/10.3390/M949