Abstract
A new compound belonging to the “heterostilbene” derivative, namely ethyl (E)-4-(2,4-dimethoxyphenyl)-6-(2,4-dimethoxystyryl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (2), has been successfully synthesized as an unprecedented side product of the Biginelli reaction between 2,4-dimethoxybenzaldehyde, ethyl acetoacetate and urea, employing PTSA as catalyst in reflux conditions and using ethanol as solvent. The molecular structure of compound (2) was established by FTIR, HRESIMS, 1D and 2D NMR.
1. Introduction
The Biginelli reaction is a multicomponent reaction used to synthesize dihydropyrimidinone (DHPM) derivatives in a one-step reaction from three components—an aldehyde, a carbonyl compound possessing the acidic C-H moiety, and urea or its derivatives—under acidic reaction conditions [1,2]. Although this reaction normally produces DHPM derivatives, there are similar reactions, usually called Biginelli-type reactions, that produce different pyrimidine derivatives, such as spiropyrimidinone [3,4] and arylidinepyrimidinone [5,6,7,8,9,10,11].
In this paper, we report a compound which differs from the product generated from both of the Biginelli-type reactions mentioned above. Despite the similarity of its reaction pattern to the Biginelli-type reaction producing arylidenepyrimidinone, there is a difference in the carbonyl component used. The aforementioned Biginelli-type reaction uses a cyclic mono carbonyl component that has two kinds of acidic C-H with equivalent reactivity, such as cyclopentanone [5,6,7,8,9,10], cyclohexanone [9,10,11] and cyclooctanone [9], so that it yields a bicyclic arylidenepyrimidinone. Interestingly, in our experiment, we used an acyclic 1,3-dicarbonyl component that possessed two acidic C-H moieties with different reactivities, namely ethyl acetoacetate. Consequently, we obtained a DHPM derivative attaching styryl moiety at C-6 (2).
2. Results and Discussion
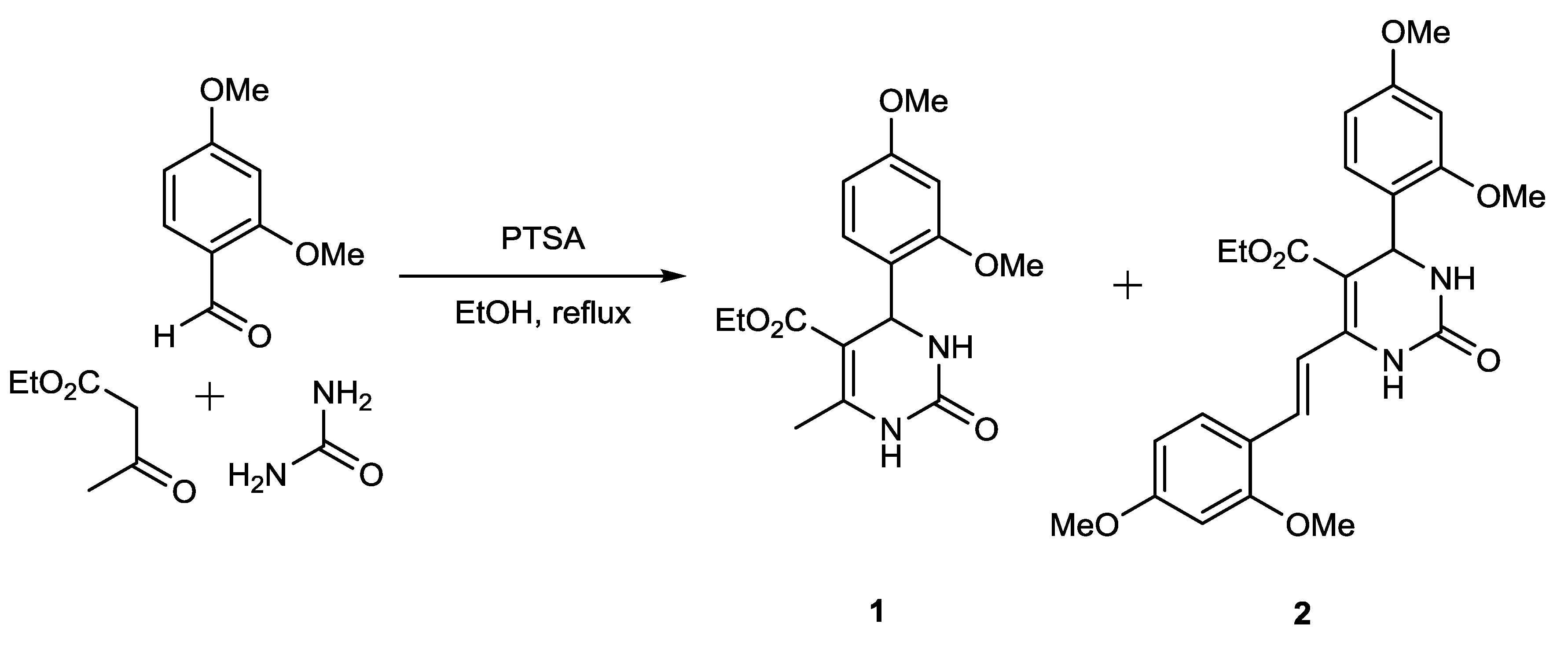
Compound 2 was isolated as a side product from the Biginelli reaction between 2,4-dimethoxybenzaldehyde, ethyl acetoacetate and urea using PTSA as catalyst in reflux condition in ethanol (Figure 1). Separation of compound 2 from the main product, namely ethyl 4-(2,4-dimethoxyphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (1), was conducted by column chromatography. Under our reaction conditions, we obtained more product 2 than product 1, although compound 2 is a side product. We got 152 mg (15.6%) of compound 1 and 402 mg (28.6%) of compound 2. Both compounds were successfully separated, their purity analyzed by TLC, and their structure then determined using spectroscopic evidence. In this paper, we do not discuss compound 1, because it has been reported previously [12].
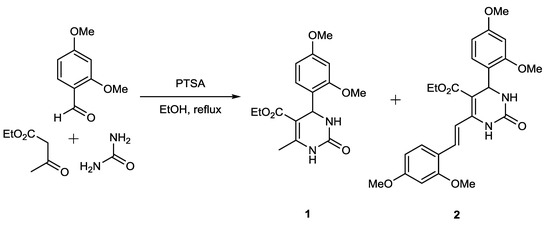
Figure 1.
Biginelli reaction producing compound 2.
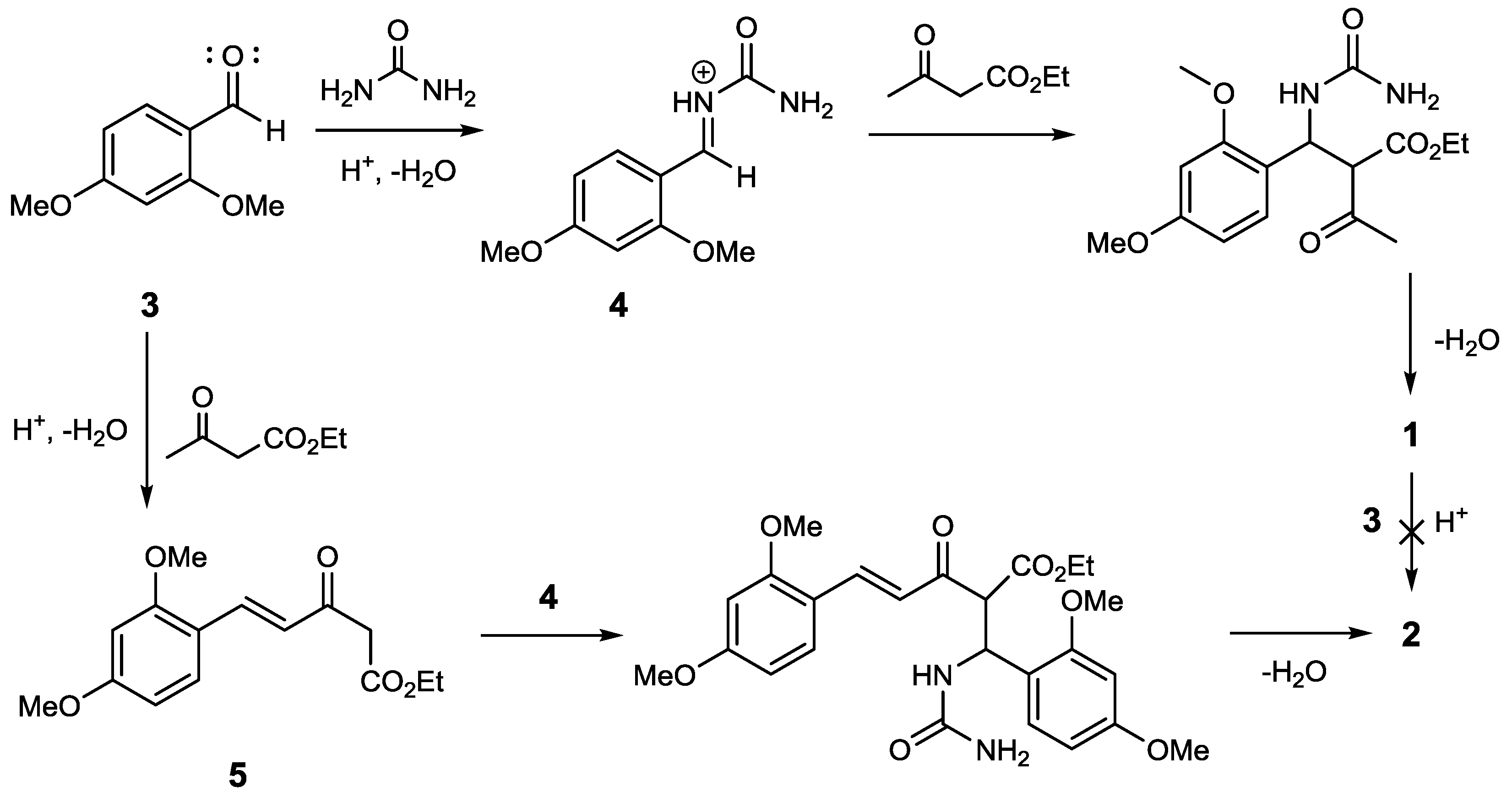
The usage of the catalyst PTSA for the Biginelli reaction has often been reported. This catalyst can be used under various reaction conditions, such as reflux in ethanol [13], grindstone [14], microwave [15] and ultrasonic irradiation [16]. However, these reaction conditions give only the main product, and do not provide side products such as compound 2. Seemingly, the amount of catalyst used has an effect on the formation of side products. The reaction condition mentioned used PTSA in a relatively low amount (<15%). In contrast with our experiment based on ethyl acetoacetate, we used 33 mol% of the catalyst. The reaction between compound 1 and 2,4-dimethoxybenzaldehyde using 33% PTSA as catalyst gave no product. This observation led to the argument that compound 2 was formed through a one-step multicomponent reaction, competing with the formation reaction of compound 1. Therefore, we propose a reaction pathway which starts with an aldol condensation between ethyl acetoacetate and 2,4-dimethoxybenzaldehyde to produce intermediate 5, which is a γ,δ-unsaturated dicarbonyl compound. A subsequent Biginelli reaction then generates compound 2 (see Figure 2).
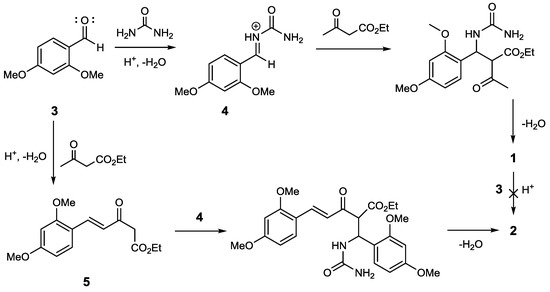
Figure 2.
Proposed reaction pathway of compound 2.
This reaction pathway differs from the pathway suggested by Zhang et al. (2015), who proposed that product 1 is an intermediate in the reaction that was conducted using the Lewis acid catalyst, FeCl3·6H2O [17]. Besides the different catalyst, the 1,3-dicarbonyl component used by Zhang was an acetoacetanilide derivative. However, our proposed reaction pathway requires further proof, because we did not verify the existence of intermediate 5 during the reaction process. In addition, the nucleophilicity of ethyl acetoacetate at the γ position is relatively low, except under strongly basic conditions, where a dianion can be formed [18,19].
Ethyl (E)-4-(2,4-dimethoxyphenyl)-6-(2,4-dimethoxystyryl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (2): pale yellow solid (402 mg, 28,6%); Rf = 0.57 (n-hexane: ethyl acetate = 1:2); HRESIMS [M − H]− calcd for C25H27N2O7 467.1818, found 467.1815; IR (DRS, KBr, cm−1): 3266, 3104 (str, NH amide), 2927 (m, CH aliphatic), 1685 (str, C=O amide, 1607 (str, C=C conjugated), 1503 (str, C=C aromatic) dan 1270 (str, Caryl-O-Calkyl); 1H-NMR (400 MHz, CDCl3) δH (ppm) 8.09 (d, J = 17.0 Hz, 1H), 7.59 (d, J = 8.6 Hz, 1H), 7.30 (d, J = 17.0 Hz, 1H), 7.01 (d, J = 8.4 Hz, 1H), 6.83 (s, 1H), 6.51 (dd, J = 8.6, 2.3 Hz, 1H), 6.46 (d, J = 2.2 Hz, 1H), 6.44 (d, J = 2.3 Hz, 1H), 6.36 (dd, J = 8.4, 2.2 Hz, 1H), 5.73 (d, J = 2.8 Hz, 1H), 5.67 (s, 1H), 4.09 (m, 2H), 3.85 (s, 3H), 3.85 (s, 3H), 3.83 (s, 3H), 3.77 (s, 3H), 1.14 (t, J = 7.1 Hz, 3H); 13C-NMR (101 MHz, CDCl3) δC (ppm) 165.8, 162.2, 160.7, 158.9, 157.9, 153.1, 145.2, 128.2, 127.7, 127.5, 122.4, 117.6, 117.4, 105.6, 103.9, 99.9, 98.9, 98.4, 60.2, 55.6, 55.6, 55.5, 55.5, 50.0, 14.3.
The HRESIMS displayed a negative molecular ion peak at m/z 467.1815, indicating a molecular formula of C25H27N2O7 and 13 degrees of unsaturation (see Supplementary Material, Figure S1). From the IR spectrum following groups N-H, the amide bond types C-H aliphatic, C=O amide type, conjugated C=C, and C-O-C alkyl-aryl ether were identified, respectively, and are exhibited by absorption band at vmax (cm−1) 3266, 2927, 1685, 1607, 1503 and 1270 (see Supplementary Material, Figure S2). Analysis of 1H-NMR (Table 1) indicating two aromatic protons with orto coupling [δH 7.59 (d, J = 8.6 Hz) and 7.01 (d, J = 8.4 Hz], two aromatic protons showing orto and meta coupling [δH 6.51 (dd, J = 8.6, 2.3 Hz) dan 6.36 (dd, J = 8.4, 2.2 Hz)], and two aromatic protons showing meta coupling [δH 6.46 (d, J = 2.2 Hz) and 6.44 (d, J = 2.3 Hz)]. This evidence indicated two aromatic rings, each possessing three protons with ABX systems. The signal of two olefinic protons, shown as two doublet signals at 8.09 and 7.30 ppm with J = 17.0 Hz, indicated the existence of an E geometric alkene. The signal at 5.73 ppm showed a benzylic or allylic proton closed to electronegative atom (nitrogen). The presence of four methoxy groups is shown by four singlet signals with an integration value of 12 at δH 3.85–3.77 ppm. The presence of multiplet signal at 4.09 ppm with an integration value of 2 and a triplet signal at 1.14 ppm with an integration value of 3 showed the existence of an ethoxy moiety possessing diastereotopic protons at CH2 moiety (see Supplementary Materials, Figures S3 and S4). In 13C-NMR (Table 1), the 25 signals shown represent all carbon atoms of compound 2 (see Supplementary Materials, Figure S5).

Table 1.
NMR data of compound 2 in CDCl3.
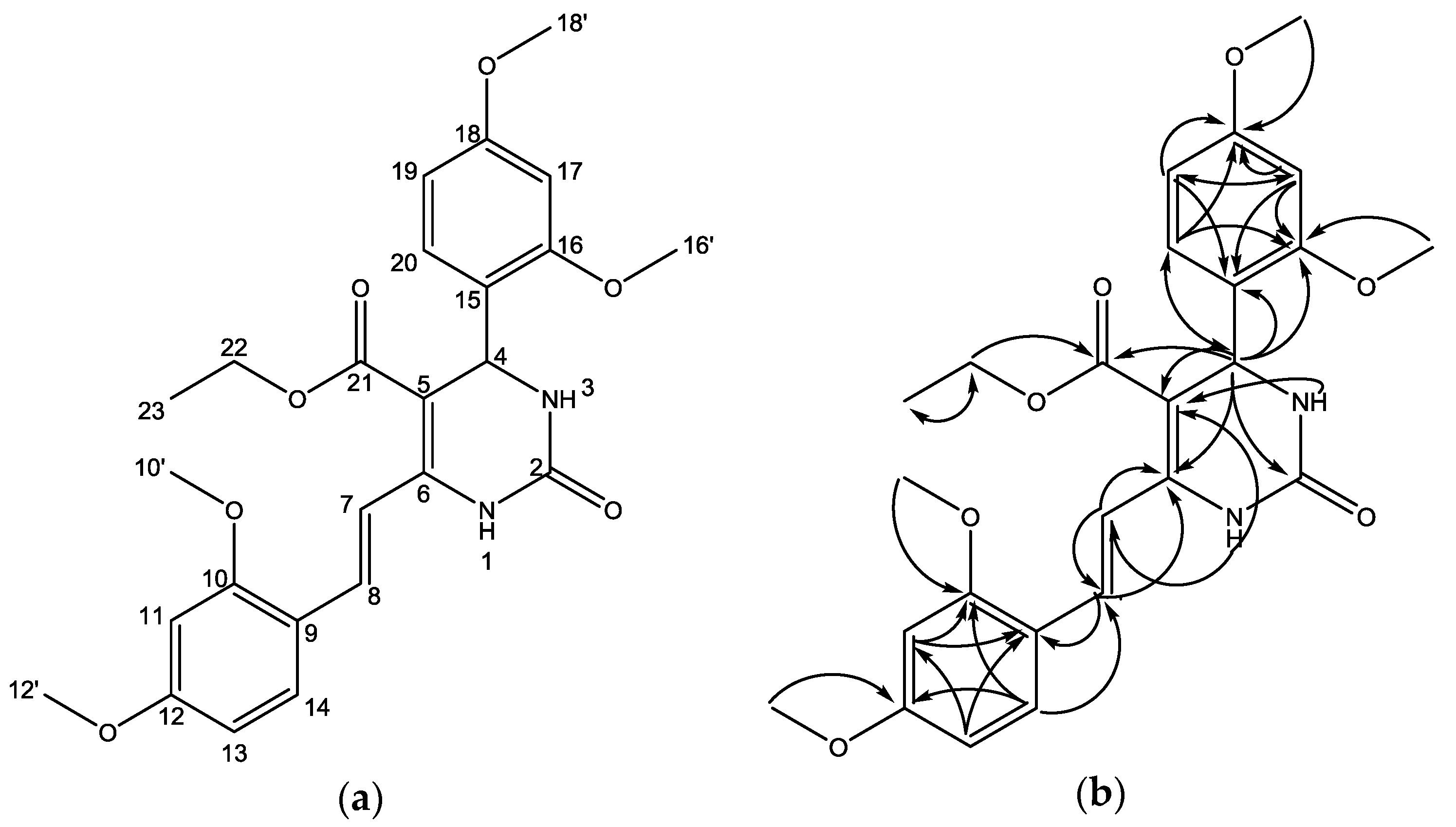
Based on the results of the HMQC experiment, we observed two protons forming no correlation with carbon atoms, namely singlet proton signal at δH at 6.83 and 5.67 ppm. This indicated that both protons were attached to a heteroatom, namely nitrogen. Furthermore, it was observed that a proton at δH 5.73 ppm attached to a carbon atom at δH 50.0 ppm (see Supplementary Materials, Figure S6). This showed that the proton is a benzylic-allylic attached to nitrogen, which is characteristic for 3,4-dihydropyrimidinone with aryl substituent at C-4. In addition, the existence of the 3,4-dihydropyrimidinone scaffold was also supported by the results of the HMBC experiment, which showed a correlation between the proton at C-4 with conjugated olefinic carbon (δC 99 ppm (C-5) and 145.2 ppm (C-6) the and urea carbonyl type (δC 153.1, C-2). The presence of the aryl group at C-4 is proved by a long-range correlation of the C-4 proton with three aromatic protons [δC 122.4 (C-15), 157.9 (C-16), and 127.5 (C-20)]. Long-range correlation of the C-4 proton with the carbon atom δC 165.8 ppm indicated that the conjugated carbonyl ester was attached to C-5. The position of styryl moiety at C-6 is proved by the long-range correlation of proton H-1 (δH 6.83 ppm) with olefinic carbon (δC 117.4. C-7). In addition, both olefinic protons [7.30 (H-7) and 8.09 (H-8)] built long-range correlations with C-6. The long-range correlations of the HMBC experiment that are possible with the structure of compound 2 are displayed in Figure 3 and in Figure S7 in the Supplementary Materials. Based on the structure elucidation, it can be concluded that compound 2 is a new compound, and it has not been previously identified in the literature.
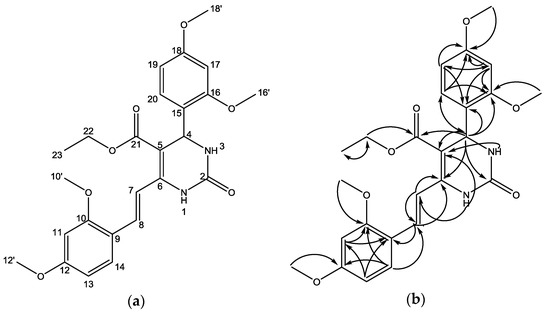
Figure 3.
(a) Numbering of the structure, and (b) Selected HMBC correlations for compound 2.
3. Materials and Methods
3.1. General
All reagents and solvents (E.Merck (Darmstadt, Germany) or Sigma Aldrich (St. Louis, MO, USA)) were used without further purification. Reaction progress was monitored by TLC on silica gel GF254 aluminum sheets (0.25 mm) using various developing systems. Spots were detected under UV light (λ 254 nm). Column chromatography was carried out using silica gel 60 G. The IR spectrum was recorded in KBr powder with the Diffuse Reflectance method on spectrophotometer IRTracer100 (Shimadzu, Kyoto, Japan). The mass spectrum was recorded using an HR mass spectrometer Waters LCT Premier XE (Waters, Santa Clara, CA, USA). The NMR spectrum (1H-, 13C-NMR, HMQC and HMBC) was recorded using JEOL 400 ECA spectrometer (JEOL, Tokyo, Japan) with CDCl3 as solvent and internal standard.
3.2. Synthesis of Compound 2
The mixture of 2,4-dimethoxybenzaldehyde (5 mmol), ethyl acetoacetate (3 mmol), urea (5 mmol), PTSA (1 mmol), and 3 mL ethanol was refluxed in a round bottom flask. The progress of the reaction was monitored by TLC. After 6 h, the reaction mixture was cooled down to room temperature, and precipitated by the addition of water. The orange precipitate (mixture of compounds 1 and 2) was then filtered off, dried, and then subjected to a silica gel 60 G column chromatography using a mixture of chloroform:ethyl acetate (3:1) as mobile phase.
4. Conclusions
A new “Heterostilbene-type” compound, namely (E)-4-(2,4-dimethoxyphenyl)-6-(2,4-dimethoxystyryl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate, is an unprecedented side product of the Biginelli reaction between 2,4-dimethoxybenzaldehyde, ethyl acetoacetate, and urea using PTSA as catalyst under reflux conditions.
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-8599/2017/3/M946, HRESIMS, FTIR, 1H-NMR, 13C-NMR, HMQC, HMBC and spectra are reported in the supplementary materials as Figures S1–S7 and structure refinement parameters as Table S1.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Authors gratefully acknowledge Lembaga Penelitian dan Inovasi, Airlangga University, for research funding through the Hibah Riset Mandat Grant 2017.
Author Contributions
H.S. brought out the ideas, managed the research, and wrote the manuscript, L.Z. and E.E. performed the synthesis, K.U.H. analyzed the data and wrote the draft. I.I. analyzed the data, A.A. and A.N.K. correct the draft.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kappe, C.O.; Stadler, A. The Biginelli dihydropyrimidine synthesis. Org. React. 2014, 63, 1–116. [Google Scholar]
- Kappe, C.O. Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc. Chem. Res. 2000, 33, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Byk, G.; Gottlieb, H.E.; Herscovici, J.; Mirkin, F. New regioselective multicomponent reaction: One pot synthesis of spiro heterobicyclic aliphatic rings. J. Comb. Chem. 2000, 2, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Abelman, M.M.; Smith, S.C.; James, D.R. Cyclic ketones and substituted α-keto acids as alternative substrates for novel Biginelli-like scaffold syntheses. Tetrahedron Lett. 2003, 44, 4559–4562. [Google Scholar] [CrossRef]
- Lei, M.; Ma, L.; Hu, L. An efficient and environmentally friendly procedure for synthesis of pyrimidinone derivatives by use of a Biginelli-type reaction. Monatshefte fur Chemie 2010, 141, 1005–1008. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Z.; Yao, Z.; Xu, F.; Shen, Q. Efficient synthesis of pyrimidinone derivatives by ytterbium chloride catalyzed Biginelli-type reaction under solvent-free conditions. Tetrahedron Lett. 2009, 50, 1622–1624. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Ghayeb, Y.; Sheikhan, N.; Ruoho, A.E. Brønsted acidic ionic liquid as an efficient and reusable catalyst for one-pot, three-component synthesis of pyrimidinone derivatives via Biginelli-type reaction under solvent-free conditions. Synth. Commun. 2011, 41, 2226–2233. [Google Scholar] [CrossRef]
- Amoozadeh, A.; Rahmani, S.; Nemati, F. Poly(ethylene)glycol/AlCl3 as a new and efficient system for multicomponent Biginelli-type synthesis of pyrimidinone derivatives. Heterocycl. Commun. 2013, 19, 69–73. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, S.; Pan, Y. Highly chemoselective multicomponent Biginelli-type condensations of cycloalkanones, urea or thiourea and aldehydes. Eur. J. Org. Chem. 2005, 2005, 2354–2367. [Google Scholar] [CrossRef]
- Wan, Y.; Yuan, R.; Xu, H.; Wang, C.; Qi, J.; Wu, H. A regioselective Biginelli-like reaction controlled by the size of alicyclic mono-ketones. J. Heterocycl. Chem. 2014, 51, E123–E128. [Google Scholar] [CrossRef]
- Ghashang, M.; Mansoor, S.S.; Aswin, K. An efficient and environmentally friendly procedure for the synthesis of some novel 8-benzylidene-4-phenyl-3,4,5,6,7,8-hexahydro-1H-quinazolin-2-ones/thiones using tetrabutylammonium hexatungstate as a reusable heterogeneous catalyst under solvent-free conditions. Bull. Korean Chem. Soc. 2013, 34, 3289–3294. [Google Scholar]
- Beşoluk, Ş.; Kucukislamoglu, M.; Zengin, M.; Arslan, M.; Nebioǧlu, M. An efficient one-pot synthesis of dihydropyrimidinones catalyzed by zirconium hydrogen phosphate under solvent-free conditions. Turk. J. Chem. 2010, 34, 411–416. [Google Scholar]
- Jin, T.; Zhang, S.; Li, T. p-Toluenesulfonic acid-catalyzed efficient synthesis of dihydropyrimidines: Improved high yielding protocol for the Biginelli reaction. Synth. Commun. 2002, 32, 1847–1851. [Google Scholar] [CrossRef]
- Bose, A.K.; Pednekar, S.; Ganguly, S.N.; Manhas, M.S. A simplified green chemistry approach to the Biginelli reaction using “Grindstone Chemistry”. Tetrahedron Lett. 2004, 45, 8351–8353. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Q.; Wang, H. Methyl 6-methyl-1-(4-methylphenyl)-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank 2012, 2012, M752. [Google Scholar] [CrossRef]
- An, L.; Han, L.; Wang, Z.; Huang, T.; Zhu, H. Calix[8]arene sulfonic acid catalyzed three-component reaction for convenient synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones under ultrasonic irradiation. Biol. Pharm. Bull. 2016, 39, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Duan, X.; Yan, X.; Yan, Y.; Liu, Q.; Liu, T.; Zhang, G. Iron-catalyzed four-member multicomponent reaction for assembly of (E)-6-arylvinyl-3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron 2015, 71, 7745–7751. [Google Scholar] [CrossRef]
- Bonne, D.; Coquerel, Y.; Constantieux, T.; Rodriguez, J. 1,3-Dicarbonyl compounds in stereoselective domino and multicomponent reactions. Tetrahedron Asymmetry 2010, 21, 1085–1109. [Google Scholar] [CrossRef]
- Jin, Y.; Roberts, F.G.; Coates, R.M. Stereoselective isoprenoid chain extension with acetoacetate dianion: (E,E,E)-geranylgeraniol from (E,E)-farnesol. Org. Synth. 2007, 84, 43–57. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).