Abstract
Ethyl 2-[2-(4-nitrobenzoyl)-1H-indol-3-yl]acetate was prepared in good yield and characterized by the aza-alkylation/intramolecular Michael cascade reaction of (E)-ethyl 3-[2-(tosylamino)phenyl]acrylate with 2-bromo-4′′-nitroacetophenone, followed by desulfonative dehydrogenation with 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) The structure of the newly synthesized compound was determined using 1H-,13C-NMR, IR and mass spectral data.
1. Introduction
Indoles are well established as privileged scaffolds, commonly encountered in many biologically active natural products and pharmaceuticals [1,2,3]. More than 10,000 biologically active indole derivatives have been identified, and more than 200 derivatives are currently known as pharmaceuticals or undergoing clinical trials [4]. Among the indoles, 2-aroyl indole-3-acetic acid derivatives, which are an important subclass of 2,3-disubstituted indoles [5], have attracted attention as a promising pro-drug for anticancer and antitumor activities [6,7]. In continuation of our research interest in 2-aminophenyl α,β-unsaturated carbonyl compounds for the synthesis of highly functionalized indole derivatives [8,9,10], we report here the preparation of a novel ethyl 2-[2-(4-nitrobenzoyl)-1H-indol-3-yl]acetate [11,12,13].
The synthesis of the title compound 4 was achieved in one-pot, as presented in Scheme 1, which was performed by the aza-alkylation/intramolecular Michael cascade reaction of (E)-ethyl 3-[2-(tosylamino)phenyl]acrylate (1) with 2-bromo-4′-nitroacetophenone (2), followed by desulfonative dehydrogenation. The reaction was carried out in acetonitrile at 60 °C in the presence of triethylamine as a base and provided the indolineinter mediate 3 within 10 min. To the resulting mixture 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) was added and gave the desired product in good yield within 20 min. The structure of compound 4 was confirmed by 1H- and 13C-NMR, IR, mass spectral data, and all data are in accordance with the assumed structure.
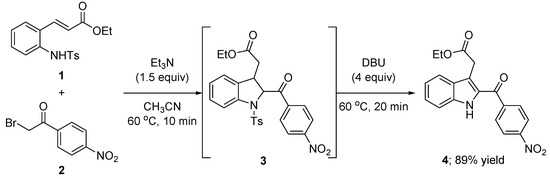
Scheme 1.
Synthesis of ethyl 2-[2-(4-nitrobenzoyl)-1H-indol-3-yl]acetate (4).
2. Experimental Section
2.1. General Information
All reagents were used as received without further purification. Chromatographic purification of the title compound 4 was accomplished using forced-flow chromatography on ICN 60 32-64 mesh silica gel 63 (Merck, Darmstadt, Germany). Thin-layer chromatography (TLC) (Merck, Darmstadt, Germany) was performed on EM Reagents 0.25 mm silica gel 60-F plates. Developed chromatograms werevisualized by fluorescence quenching and anisaldehyde stain. 1H- and 13C-NMR spectra were recorded on a 400 MHz instrument (Bruker BioSpin GmbH, Karlsruhe, Germany) as noted, and are internally referenced to residual protio solvent signals. Data for 1H-NMR are reported as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet), integration, coupling constant (Hz) and assignment. Data for 13C-NMR are reported in terms of chemical shift. IR spectra were recorded on a Perkin-Elmer 1600 FT-IR spectrometer (Bruker Optics GmbH, Ettlingen, Germany), and reported in terms of frequency of absorption (cm−1). High-resolution mass spectrometry data were recorded on a JEOL JMS-700 MStation mass spectrometer (JEOL, Tokyo, Japan).
2.2. Syntheis of ethyl 2-[2-(4-nitrobenzoyl)-1H-indol-3-yl]acetate(4)
To a solution of (E)-ethyl 3-[2-(tosylamino)phenyl]acrylate (1, 35 mg, 0.10 mmol, 1.0 equiv) in CH3CN (0.5 mL), 2-bromo-4′-nitroacetophenone (2, 37 mg, 0.15 mmol) and Et3N (21 μL, 0.15 mmol,) were added at room temperature. After stirring the resulting mixture for 10 min at 60 °C, DBU (60 μL, 0.40 mmol) was added. The resulting mixture was stirred further for 20 min at 60 °C, and saturated aqueous NaHCO3 was added. The mixture was then extracted with EtOAc. The combined organic layer was washed with brine, dried over anhydrous MgSO4, and concentrated in vacuo.The crude product waspurified on silica gel column chromatography using ethyl acetate and hexane (1/10) as eluents to afford the desired title compound 4 (89%, 31 mg).White solid; m.p. 163–165 °C; 1H-NMR (400 MHz, CDCl3) δ 9.18 (s, 1H), 8.31 (d, J = 8.7 Hz, 2H), 7.85 (d, J = 8.7 Hz, 2H), 7.64 (d, J = 8.1 Hz, 1H), 7.45–7.34 (m, 2H), 7.19 (ddd, J = 8.0, 5.1, 2.8 Hz, 1H), 4.11 (q, J = 7.1 Hz, 2H), 3.76 (s, 2H), 1.23 (t, J = 7.1 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ 186.46, 170.80, 149.62, 144.18, 136.83, 131.36, 129.69, 128.16, 127.29, 123.72, 121.30, 121.13, 117.51, 112.44, 61.23, 31.16, 14.17.; IR (film) 3322, 2934, 1722, 1616, 1598, 1522, 1433, 1370, 1331, 1257, 1215, 1170, 1103, 1028, 1008, 990 cm−1; HRMS (EI) m/z calcd for [M]+ C19H16N2O5: 352.1059 Found: 352.1051.
Supplementary Materials
1H- and13C-NMR spectra for compound 4 are available online.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
This work was supported by Kyonggi University's Graduate Research Assistantship 2017.
Author Contributions
Both authors contributed equally to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical importance of indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine Indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Gul, W.; Hamann, M.T. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005, 78, 442–543. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, K.C.; Chattopadhyay, S.K. Heterocycles in Natural Product Synthesis; Wiley-VCH: Weinheim, Germany, 2011; pp. 221–265. [Google Scholar]
- Brancale, A.; Silvestri, R. Indole, a core nucleus for potent inhibitors of tubulin polymerization. Med. Res. Rev. 2007, 27, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Folkes, L.K.; Wardman, P. Oxidative activation of indole-3-acetic acids to cytotoxic species—A potential new role for plant auxins in cancer therapy. Biochem. Pharm. 2001, 61, 129–136. [Google Scholar] [CrossRef]
- Wardman, P. Indole-3-acetic acids and horseradish peroxidase: A new prodrug/enzyme combination for targeted cancer therapy. Curr. Pharm. Des. 2002, 8, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Kim, C.; Kim, S.-G. One-pot synthesis of functionalized 2,3-disubstituted indolines via K2CO3-promoted cascade Michael/aza-cyclization reactions. Bull. Korean Chem. Soc. 2015, 36, 417–420. [Google Scholar] [CrossRef]
- Yu, M.; Kim, S.-G. Stereoselective synthesis of cis-2,3-disubstituted indolines via an aza-alkylation/Michael cascade reaction. Tetrahedron Lett. 2015, 56, 7034–7037. [Google Scholar] [CrossRef]
- Kim, A.; Yu, M.; Sim, J.-T.; Kim, S.-G. One-pot synthesis of 2-acylindole-3-acetylketones via domino aza-alkylation/Michael reaction using o-aminophenyl α,β-unsaturated ketones followed by desulfonative dehydrogenation. Bull. Korean Chem. Soc. 2016, 37, 1529–1532. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Cruz-Lopez, O.; Carrion, M.D.; Cara, C.L.; Balzarini, J.; Fabbri, E.; Gambari, R. Synthesis and biological evaluation of a series of 2-(3,4,5-trimethoxybenzoyl)-indol-3-yl acetic acid derivatives as potential agents against human leukemia K562 cells. Lett. Drug Des. Descov. 2008, 5, 214–220. [Google Scholar] [CrossRef]
- Nakao, K.; Murata, Y.; Koike, H.; Uchida, C.; Kawamura, K.; Mihara, S.; Hayashi, S.; Stevens, R.W. Synthesis of 2-acylindole-3-acetic acids: A novel base-mediated indole synthesis. Tetrahedron Lett. 2003, 44, 7269–7271. [Google Scholar] [CrossRef]
- Caron, S.; Vazquez, E. Efficient synthesis of [6-chloro-2-(4-chlorobenzoyl)-1H-indol-3-yl]acetic acid, a novel COX-2 inhibitor. J. Org. Chem. 2003, 68, 4104–4107. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).