Abstract
Ethyl({[acryloyl(furan-2-ylmethyl)amino]acetyl}amino)acetate was synthesized via Ugi four component (4C) reaction at ambient temperature. The protocol employs a reaction between formaldehyde, furfurylamine, acrylic acid, and ethyl 2-isocyanoacetate. The course of the reaction was found to be high yielding, and the resulting glycine ester derivative was well characterized by elemental analysis, FTIR, NMR spectroscopy, and mass spectrometric techniques.
1. Introduction
Proteins and peptides have been an important class of biomolecules since the evolution of modern life. They are the central framework of all biological events governing aspects of structure, transport, and catalytic pathways in nature [1]. Created intricately in a highly specific fashion, the functions of proteins and peptides in the living system have been a wonder and an inspiration in synthetic chemistry [2].
Peptoids are structural isomers of natural peptides where the side chain functionality is joined to the nitrogen atom of the peptide backbone rather than to the α-carbons (as they are in peptides). This new class of non-natural molecules mimics the natural structure of peptides and has versatile biological activities, and many of the classic disadvantages of native peptides have been circumvented by their development [3,4]. It should, however, be noted that, because of their remarkable proteolytic stability, exploration leads to a modular drug discovery approach by combinatorial synthesis [5,6].
Low-molecular-weight gelators (LMWGs) are small organic compounds that can self-assemble to form fibrous supramolecular structures in aqueous/organic media. Recently, the divergent synthesis of tripeptoids can be seen as a combinatorial approach to LMWGs by a one-pot Ugi multicomponent reaction from simple starting materials [7,8]. Several organic compounds especially amino acids, cholesterols, sugars, and ureas, are classic examples for the design of these LMWGs. Significant efforts have been made by researchers to exploit the spontaneous molecular arrangement of peptides/peptoids and their derivatives in aqueous/organic solutions, and to afford nanostructured hydrogels under specific conditions. These highly hydrated scaffolds have potential applications in 3D cell culture and tissue engineering [9,10].
With concern over the increasing demand for substituted alkyl acrylates, many researchers have designed various synthons from aza-Michael addition reactions [11,12] and reported their gelation properties. The present paper describes the synthesis of an acryl functional tripeptoid with a furan moiety via Ugi four component (4C) reaction at ambient temperature. Moreover, the reported title compound can be utilized as a starting material for the aza-Michael addition reaction.
2. Results and Discussion
Ethyl({[acryloyl(furan-2-ylmethyl)amino]acetyl}amino)acetate was synthesized via Ugi four component (4C) reaction involving furfurylamine, paraformaldehyde, acrylic acid, and ethyl 2-isocyanoacetate at ambient conditions for 30 h, as shown in Scheme 1. The target compound is yellow, naturally oily, insoluble in water and non-polar solvents such as n-hexane, and completely soluble in dichloromethane, chloroform, methanol, ethanol, and dimethyl sulfoxide.
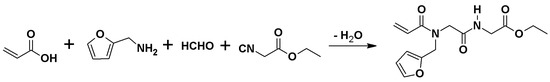
Scheme 1.
Synthesis of ethyl({[acryloyl(furan-2-ylmethyl)amino]acetyl}amino)acetate.
The 1H- &13C-NMR of the title compound is presented in Figure 1 and Figure 2. The data revealed that the peak at 7.38 δ is for the aromatic proton of the furan ring (12H). The peaks in the range 6.77–6.80 δ corresponds to CH of the acryloyl group [H2C=CH–C(=O)–]. The signals for the aromatic protons of the furan ring (13H, 14H) along with CH2 of the acryloyl group [H2C=CH–C(=O)–] have been attributed to the peaks in the range of 6.34–6.47 δ. The δ value in the range 5.10 corresponds to the CH2 connected to the furan ring (10H). The δ value in the range 4.58–4.71 ascribed to CH2 of the ester functionality (8H). The peak at 3.98 δ and 2.99 δ corresponds to 4H and 6H protons, respectively. Finally, the peak at 1.26–1.27 δ corresponds to methyl protons (9H).
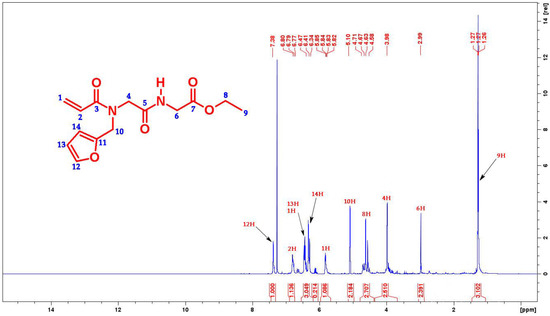
Figure 1.
1H-NMR spectra of the title compound (700 MHz, CDCl3) at room temperature.
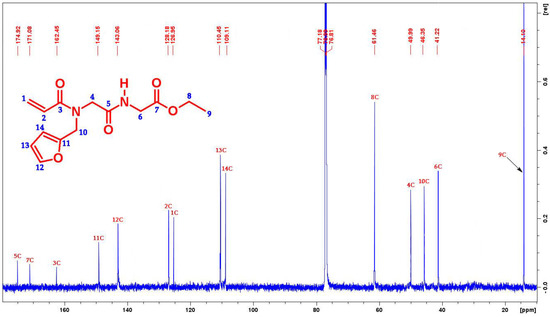
Figure 2.
13C-NMR spectra of the desired compound (176 MHz, CDCl3) at room temperature.
3. Experimental Section
3.1. Materials
Furfurylamine ≥99%, ethyl 2-isocyanoacetate, paraformaldehyde, acrylic acid, aluminum oxide (Ultra dry powder, Type—Neutral), and methanol were procured from Sigma-Aldrich, Prague, Czech Republic. All other chemicals and solvents were of AR grade and used without further purification. Double distilled water was used throughout the study.
3.2. Instrumentaion
The 1H- &13C-NMR spectra were recorded on a 700 MHz Bruker Avance III HD spectrometer with a 5 mm dual broad-band probe, a 5 mm dual inverse broad-band probe, and a 1.7 mm triple resonance (1H–13C) probe (Bruker, Leiderdorp, The Netherlands) for multinuclear applications in liquids and solids. Tetramethylsilane (TMS) was used as an internal standard. LCMS measurement was performed on a liquid chromatography system with mass spectrometry detection (Q-TOF)—Agilent ACCURATE MASS 6530 Q TOF LC/MS (Agilent Technologies, Santa Clara, CA, USA). The elemental analysis was carried out on a VARIO EL III Elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany), and the results for C, H, and N were within 0.4% of the theoretical values. FTIR analysis was carried out on Nicolet 6700 (ATR, Thermo Scientific, Waltham, Middlesex County, MA, USA) in scanning range 4000–600 cm−1. The resolution was 4 cm−1 with 64 scans, and the measurement was performed with germanium crystal.
3.3. Synthesis of Ethyl({[acryloyl(furan-2-ylmethyl)amino]acetyl}amino)acetate
To a 50 mL of round bottomed flask, paraformaldehyde (0.042 g, 1.38mmol) was added to a stirring solution of furfurylamine (100 mg, 1.38 mmol) in methanol (10 mL). The solution was stirred at room temperature for 2 h. Later, acrylic acid (0.1 g, 1.38mmol) was added, followed by ethyl 2-isocyanoacetate (0.156 g, 1.38 mmol). The completion of the reaction was monitored using thin-layer chromatography (TLC) analysis (performed with Merck Kieselgel TLC aluminum oxide 60 F254, neutral TLC plates, Merck Millipore, Bangalore, India). The formation of the product was confirmed by after 30 h. The reaction mixture was diluted with 20 mL of ethyl acetate (EtOAc) and washed with water (15 mL × 4) and, afterwards, a saturated solution of brine (20 mL). The collected organic layer was dried over anhydrous sodium sulfate, and solvent was removed under reduced pressure to collect the crude product (0.385 g). The crude product was passed through neutral alumina using 45% EtOAc/n-hexane. The desired product was obtained as a yellow oily liquid (0.295 g) in a 72% yield. The supporting FTIR and LCMS spectra are presented in the supplementary material file.
13C-NMR (176 MHz, CDCl3): (ppm) 174.92, 171.08, 162.45, 149.15, 143.06, 128.18, 126.95, 110.45, 109.11, 61.46, 49.99, 46.35, 41.22, 14.01. Anal. calcd. for C14H18N2O5 (294.303): C, 57.13; H, 6.16; N, 9.52. Found: C, 56.81; H, 5.76; N, 9.58. LCMS (ESI): m/z = 295.127 (Found), 294.303 (Expected for [M + H]+), FTIR (Ge Crystal): 3385, 3180, 2985 cm−1 (-NH Stretch), 1686, 1744 cm−1 (C=O); 1125 cm−1 (C-O-C).
Supplementary Materials
LCMS and FTIR spectra for the title compound are available online at www.mdpi.com/1422-8599/2017/1/M925.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
The work is supported by MŠMT ČR-USA Kontakt II (LH14050) and the Molecular Foundry, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. This research is also supported in part by a grant from the Ministry of Education, Youth and Sports of the Czech Republic - NPU Program I (LO1504). We also acknowledge Pavel Kucharczyk, Centre of Polymer Systems, University Institute, Tomas Bata University in Zlin, Tř. T. Bati 5678, 760 01, Zlin, Czech Republic, for LCMS measurement.
Author Contributions
S.D.G. designed the synthesis, carried out the experiments for the title compound synthesis, and wrote the article. N.S. and P.S. confirmed the data analysis. R.N.Z. helped in the discussion of the results. All of the above authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gutteridge, A.; Thornton, J.M. Understanding nature’s catalytic toolkit. Trends Biochem. Sci. 2005, 30, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Reddington, S.C.; Howarth, M. Secrets of a covalent interaction for biomaterials and biotechnology: SpyTag and SpyCatcher. Curr. Opin. Chem. Biol. 2015, 29, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.J.; Kania, R.S.; Zuckermann, R.N.; Huebner, V.D.; Jewell, D.A.; Banville, S.; Ng, S.; Wang, L.; Rosenberg, S.; Marlowe, C.K.; et al. Peptoids: A modular approach to drug discovery. Proc. Natl. Acad. Sci. USA 1992, 89, 9367–9371. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N. Peptoid Origins. Biopolymers (PeptSci) 2011, 96, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N.; Kodadek, T. Peptoids as potential therapeutics. Curr. Opin. Mol. Ther. 2009, 11, 299–307. [Google Scholar] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Mangunuru, H.P.R.; Yang, H.; Wang, G. Synthesis of peptoid based small molecular gelators by a multiple component reaction. Chem. Commun. 2013, 49, 4489–4491. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.N.; Das, D.; Roy, S.; Das, P.K. Structure and Properties of Low Molecular Weight Amphiphilic Peptide Hydrogelators. J. Phys. Chem. B 2007, 111, 14107–14113. [Google Scholar] [CrossRef] [PubMed]
- Worthington, P.; Pochan, D.J.; Langhans, S.A. Peptide Hydrogels—Versatile Matrices for 3D Cell Culture in Cancer Medicine. Front. Oncol. 2015, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Biswas, G.; Moon, H.J.; Boratyński, P.; Jeong, B.; Kwon, Y.-U. Structural sensitivity of peptoid-based low molecular mass organogelator. Mater. Des. 2016, 108, 659–665. [Google Scholar] [CrossRef]
- Nigam, M.; Rush, B.; Patel, J.; Castillo, R.; Dhar, P. Aza-Michael Reaction for an Undergraduate Organic Chemistry Laboratory. J. Chem. Educ. 2016, 93, 753–756. [Google Scholar] [CrossRef]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).