Abstract
4,6-Dichloro-2-(methylthio)pyrimidine (3) reacts with EtONa in EtOH, at ca. 20 °C, for 2 h, to give exclusively 4-chloro-6-ethoxy-2-(methylthio)pyrimidine (5) in 89% yield. The latter is presented as a useful multifunctionalised pyrimidine scaffold.
1. Introduction
Pyrimidines are well known owing to their presence in biological systems as components of the nucleic acid bases cytosine, thymine, and uracil, as well as the vitamin thiamine, which illustrates their importance. The presence of pyrimidines has been reported for over a century and their chemistry has been reviewed [1]. Pyrimidines also find a plethora of uses as pharmaceuticals, as anti-inflammatory [2], anti-microbial [3], anti-HIV [4], anti-malarial [5] and anti-tumour [6] agents.
As part of our ongoing work in the chemistry of 1,2,6-thiadiazines [7,8,9,10] we identified 4,5,6-trichloropyrimidine-2-carbonitrile (1) as a product of the degradation of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine (2) (Scheme 1) [8]. The former was also previously identified as a minor side product from the reaction of tetracyanoethene (TCNE) with SCl2 [11].
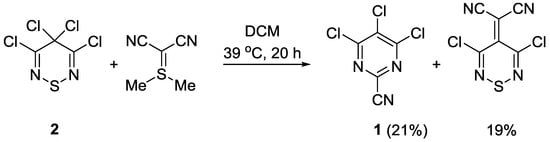
Scheme 1.
Isolation of trichloropyrimidine 1 from 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine (2) [11].
Polyhalogenated pyrimidines are useful scaffolds as they can be modified either by nucleophilic aromatic substitution or by palladium coupling reactions. However, these reactions often suffer from regioselectivity issues. For example, the Suzuki-Miyaura coupling of 2,3,4,5-tetrachloropyrimidine with arylboronic acids gives either the 2- [12] or the 4-arylpyrimidine [13]. Moreover, on the same scaffold, while nucleophilic substitution by alkoxide occurs at C2 [14], hydroxide attacks the C4 position [15]. On the other hand, nucleophilic substitution by amine nucleophiles often gives mixtures of products [16,17]. The complexity of the chemistry of pyrimidines means that it is often difficult to access specific pyrimidine targets from a simple and symmetrical pyrimidine scaffold, indicating the necessity for asymmetrical and sometimes multi-functionalized scaffolds.
2. Results and Discussion
In our efforts to develop an independent synthesis for the trichloropyrimidine 1 we prepared the known 4,6-dichloro-2-(methylthio)pyrimidine (3) as an early and readily available [18] intermediate towards the preparation of 3,4,5-trichloro-2-(methylthio)pyrimidine (4). Nevertheless, attempts to chlorinate the C5 position, using either NCS or PCl5 to obtain 3,4,5-trichloro-2-(methylthio)pyrimidine (4) all failed. As such, we attempted to increase the electron density at C5 to facilitate the desired chlorination. To do this, we introduced an ethoxy group via simple nucleophilic aromatic substitution of chlorine. Interestingly, treating the dichloropyrimidine 3 with EtONa (1.1 equiv.) in EtOH proceeded smoothly at ca. 20 °C to give exclusively the mono-displaced 4-chloro-6-ethoxy-2-(methylthio)pyrimidine (5), but this disappointingly also failed to undergo C5 chlorination to give the expected 5,6-dichloro-4-ethoxy-2-methylthiopyrimidine (6) (Scheme 2).

Scheme 2.
Synthesis of 4-chloro-6-ethoxy-2-(methylthio)pyrimidine (5) and attempted synthesis of 5-chlorinated pyrimidines 4 and 6.
Despite this setback, we note that ethoxypyrimidine 5 is potentially a useful pyrimidine scaffold. Pyrimidine 5 has been previously prepared by treatment of dichloropyrimidine 3 with NaOEt in dry dimethylformamide, at 70 °C overnight [19], however, the synthesis described herein uses a non-toxic solvent (EtOH) and milder conditions (20 °C) that are more suitable for a regioselective reaction. A very similar analogue 4-chloro-6-methoxy-2-(methylthio)pyrimidine [20] is a versatile scaffold and can undergo Suzuki-Miyaura coupling to afford 4-aryl-6-methoxy-2-(methylthio)pyrimidines [21], as well as, amine [20] or alcohol displacement of the chloride [22], oxidation to sulfone [23], and condensation reactions to give polycyclic systems [24,25].
3. Materials and Methods
The reaction mixture was monitored by chromatography (TLC) using commercial glass backed TLC plates (Merck Kieselgel 60 F254, Darmstadt, Germany). The plates were observed under UV light at 254 and 365 nm. The melting point was determined using a PolyTherm-A, Wagner and Munz, Kofler hot-stage microscope apparatus (Wagner and Munz, Munich, Germany). The solvent used for recrystallization is indicated after the melting point. The UV-VIS spectrum was obtained using a Perkin-Elmer Lambda-25 UV-VIS spectrophotometer (Perkin-Elmer, Waltham, MA, USA) and inflections are identified by the abbreviation “inf”. The IR spectrum was recorded on a Shimadzu FTIR-NIR Prestige-21 spectrometer (Shimadzu, Kyoto, Japan) with Pike Miracle Ge ATR accessory (Pike Miracle, Madison, WI, USA) and strong, medium, and weak peaks are represented by s, m, and w, respectively. 1H- and 13C-NMR spectra were recorded on a Bruker Avance 500 machine (at 500 and 125 MHz, respectively (Bruker, Billerica, MA, USA)). Deuterated solvents were used for homonuclear lock and the signals are referenced to the deuterated solvent peaks. APT (Advance Proton Test) NMR studies identified carbon multiplicities, which are indicated by (s), (d), (t) and (q) notations. The MALDI-TOF (Matrix Assisted Laser Desorption/Ionization-Time of Flight) mass spectrum (+ve mode) was recorded on a Bruker Autoflex III Smartbeam instrument (Bruker). The elemental analysis was run by the London Metropolitan University Elemental Analysis Service. 4,6-Dichloro-2-(methylthio)pyrimidine (3) was prepared according to the literature procedure [18].
4-Chloro-6-ethoxy-2-(methylthio)pyrimidine (5)
To a stirred mixture of 4,6-dichloro-2-(methylthio)pyrimidine (3) (50 mg, 0.256 mmol) in EtOH (1 mL) at ca. 20 °C was added dropwise to a freshly prepared solution of EtONa (0.28 mL, 0.28 mmol, 1 M in EtOH). The mixture was protected with a CaCl2 drying tube and stirred at this temperature until complete consumption of the starting material (TLC, 2 h). DCM (10 mL) was then added, followed by a saturated aqueous solution of NaHCO3 (10 mL) and the mixture extracted. The aqueous phase was then extracted with a further 10 mL of DCM. The combined organic phases were dried (Na2SO4), filtered and evaporated under vacuum to yield 4-Chloro-6-ethoxy-2-(methylthio)pyrimidine 5 (46.6 mg, 89%) as colourless needles, m.p. 59–60 °C (from n-pentane, −60 °C); Rf 0.16 (n-hexane/DCM, 90:10); (found: C, 41.13; H, 4.36; N, 13.60. C7H9ClN2OS requires C, 41.08; H, 4.43; N, 13.69%); λmax (DCM)/nm 276 inf (log ε 3.78), 255 (4.06); vmax/cm−1 3007w (aryl C-H), 2982w, 2934w, 2888w and 2849w (alkyl C-H), 1562s, 1557m, 1541s, 1537s, 1429m, 1420w, 1379m, 1352m, 1333m, 1323m, 1317m, 1273s, 1221w, 1038s, 989w, 833w, 870m, 814s; δH (500 MHz; CDCl3) 6.37 (1H, s, Ar H), 4.41 (2H, q, J 7.1, OCH2), 2.52 (3H, s, SCH3), 1.37 (3H, t, J 7.1, CH2CH3); δC (125 MHz; CDCl3) 172.7 (s), 169.4 (s), 160.2 (s), 102.4 (d), 63.3 (t), 14.2 (q), 14.1 (q); m/z (MALDI-TOF) 206 (M+ + 2, 53%), 204 (M+, 100), 176 (52), 160 (59).
Supplementary Materials
The following are available online at www.mdpi.com/1422-8599/2017/1/M923.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
The University of Cyprus Grant “Post-doctoral Researchers” supporting Andreas Kalogirou is greatly acknowledged. The authors thank the Cyprus Research Promotion Foundation (Grant: NEKYP/0308/02) and the following organizations and companies in Cyprus for generous donations of chemicals and glassware: the State General Laboratory, the Agricultural Research Institute, the Ministry of Agriculture, MedoChemie Ltd, Medisell Ltd and Biotronics Ltd. Furthermore, we thank the A. G. Leventis Foundation for helping to establish the NMR facility at the University of Cyprus.
Author Contributions
P.A. Koutentis conceived the experiments; A.S. Kalogirou designed and performed the experiments, analyzed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Rewcastle, G.W. Pyrimidines and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Pergamon Press: Oxford, UK, 2008; Volume 8, pp. 120–252. [Google Scholar]
- Amr, A.E.; Nermien, M.S.; Abdulla, M.M. Synthesis, reactions, and anti-inflammatory activity of heterocyclic systems fused to a thiophene moiety using citrazinic acid as synthon. Monatsh. Chem. 2007, 138, 699–707. [Google Scholar] [CrossRef]
- Desai, K.; Patel, R.; Chikhalia, K. Synthesis of pyrimidine based thiazolidinones and azetidinones: Antimicrobial and antitubercular agents. Ind. J. Chem. 2006, 45, 773–778. [Google Scholar]
- Fujiwara, N.; Nakajima, T.; Ueda, Y.; Fujita, H.; Kawakami, H. Novel piperidinylpyrimidine derivatives as inhibitors of HIV-1 LTR activation. Bioorg. Med. Chem. 2008, 16, 9804–9816. [Google Scholar] [CrossRef] [PubMed]
- Gorlitzer, K.; Herbig, S.; Walter, R.D. Indeno[1,2-d]pyrimidin-4-yl-amines. Pharmazie 1997, 52, 670–672. [Google Scholar]
- Wagner, E.; Al-Kadasi, K.; Zimecki, M.; Sawka-Dobrowolska, W. Synthesis and pharmacological screening of derivatives of isoxazolo[4,5-d]pyrimidine. Eur. J. Med. Chem. 2008, 43, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Manoli, M.; Koutentis, P.A. Synthesis of N-aryl-3,5-dichloro-4H-1,2,6-thiadiazin-4-imines from 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine. Org. Lett. 2015, 17, 4118–4121. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Koutentis, P.A. A qualitative comparison of the reactivities of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine and 4,5-dichloro-1,2,3-dithiazolium chloride. Molecules 2015, 20, 14576–14594. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Koutentis, P.A. Regioselective geminal dichloride reactivity of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine: access to 4,4-dioxo- and dithio-ketals. Tetrahedron Lett. 2016, 57, 203–205. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. Substitution chemistry of 3,5-dichloro-4H-1,2,6-thiadiazine 4,4-ketals. Tetrahedron Lett. 2016, 57, 3307–3310. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. J. Chem. Soc. Perkin Trans. 1 2000, 1089–1094. [Google Scholar] [CrossRef]
- Hyun, S.Y.; Jung, S.O.; Lee, R.N. An Electroluminescent Compound and an Electroluminescent Device Comprising the Same. KR2015/124637 A, 25 November 2015. [Google Scholar]
- Liu, J.; Fitzgerald, A.; Mani, N. Facile assembly of fused benzo[4,5]furo Heterocycles. J. Org. Chem. 2008, 73, 2951–2954. [Google Scholar] [CrossRef] [PubMed]
- Mabuko, Y. Triallylisocyanurate, Triallylcyanurate, and Process for Production of Triallylisocyanurate. EP2436677 A1, 4 April 2012. [Google Scholar]
- Chen, Y.; Kanouni, T.; Kaldor, S.; Stafford, J.A.; Veal, J.M. Inhibitors of Lysine Specific Demethylase-1. WO2015/168466 A1, 5 November 2015. [Google Scholar]
- Ladd, D.L. Synthesis of some substituted guanidinopyrimidines and their structural assignment by 13C and 1H-NMR. J. Het. Chem. 1982, 19, 917–921. [Google Scholar] [CrossRef]
- Kukla, M.J.; Ludovici, D.W.; Kavash, R.W.; de Corte, B.L.D.; Heeres, J.; Janssen, P.A.J.; Koymans, L.M.H.; de Jonge, M.R.; van Aken, K.J.A.; Krief, A. Prodrugs of HIV Replication Inhibiting Pyrimidines. EP1282607 B1, 12 February 2003. [Google Scholar]
- Raboisson, P.; Belfrage, A.; Classon, B.; Lindquist, K.; Nilsson, K.; Rosenquist, A.; Samuelson, B.; Wahling, H. Pyrimidine Substituted Macrocyclic HCV Inhibitors. WO2008/095999A1, 14 August 2008. [Google Scholar]
- Bayne, C.D.; Johnson, A.T.; Lu, S.-P.; Mohan, R.; Nyman, M.C.; Schweiger, E.J.; Stevens, W.C.; Wang, H.; Xie, Y. Modulators of LXR. US20050080111 A1, 14 April 2005. [Google Scholar]
- Seganish, W.M.; Fischmann, T.O.; Sherborne, B.; Matasi, J.; Lavey, B.; McElroy, W.T.; Tulshian, D.; Tata, J.; Sondey, C.; Garlisi, C.G.; et al. Discovery and Structure Enabled Synthesis of 2,6-Diaminopyrimidin-4-one IRAK4 Inhibitors. ACS Med. Chem. Lett. 2015, 6, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.W.; Lai, C.; Miyashiro, J.M.; Tong, Y.; Florjancic, A.S.; Han, E.K.; Soni, N.; Shi, Y.; Lasko, L.; Leverson, J.D.; et al. Aminopyrimidinone Cdc7 Kinase Inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 1940–1943. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, Y.; Sanemitsu, Y. Uracil Compounds and Use Thereof. US6537948 B1, 25 March 2003. [Google Scholar]
- Kanno, H.; Kubota, Y.; Sato, T.; Arahira, M. 2-Benzyloxy-4-phenoxypyrimidine Derivative, Processes for Producing the Derivative and Herbicidal Composition Containing the Derivative. US5723412 A1, 3 March 1998. [Google Scholar]
- Kobayashi, K.; Ono, R.; Yuba, S.; Hiyoshi, H.; Umezu, K. Two-step synthesis of 5-hydroxy-5,7-dihydro-6H-pyrrolo[2,3-d]pyrimidin-6-one derivatives from 4-chloro-6-methoxy-2-(methylsulfanyl)pyrimidine. Heterocycles 2015, 91, 1177–1185. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kuroda, M.; Tanaka, N.; Yokoi, Y.; Kobayashi, A.; Hiyoshi, H.; Umezu, K. A simple method for the preparation of pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dithione derivatives. Heterocycles 2014, 89, 1933–1939. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).