Abstract
Starting from 1-(2-fluorophenyl)-1,3-dihydro-2H-benzimidazol-2-one (1) and (1-bromo-3-chloropropyl)benzene (2), the target compound 3, which represents a precursor for future radiolabeling, is prepared in a three-step synthesis.
Keywords:
NET, PET, FAPPI 1. Introduction
The norepinephrine transporter (NET) plays a pivotal role in a variety of diseases, which not only include neurological/psychiatric disorders [1,2], but also cardiovascular [1,2,3] and metabolic diseases [3,4,5]. Thus, the investigation of the underlying dysregulation-mechanism of the noradrenergic system is of major interest.
As a non-invasive molecular imaging technique, positron emission tomography (PET) is the most suitable technique today to gain information about the transporter abundance and density in healthy and pathological living human brains [6]. It represents an accurate approach towards the collection of missing data in the living organism and also enables direct quantification of receptor and transporter densities in vivo. To fully gain insight into the molecular changes of the noradrenergic system via PET, however, prior development of suitable NET-PET radioligands is required. Currently, only PET tracers derived from reboxetine have been used in clinical studies [7] displaying certain limitations in their applicability, e.g. due to metabolic considerations [8].
On the basis of 11C-radiolabeled 1-(3-(methylamino)-1-phenylpropyl)-3-phenyl-1,3-dihydro-2H-benzimidazole-2-one ([11C]Me@APPI) [9], which represents a suitable NET radioligand for use in PET, recently several non-radiolabeled analogs of [11C]Me@APPI have been described as potential reference compounds for PET based investigations of the NET [10]. In continuation of these previous studies, we are reporting in this paper the synthesis of 1-(3-amino-1-phenylpropyl)-3-(2-fluorophenyl)-1,3-dihydro-2H-benzimidazol-2-one (3), a precursor molecule for future radiolabeling operations.
2. Results and Discussion
As previously published, derivative 1 was made accessible by reacting 1-fluoro-2-nitrobenzene with 2-fluoroaniline, followed by reduction of the nitro group. Subsequent cyclization with 1,1′-carbonyldiimidazole then afforded 1 [10]. For the preparation of side chain 2, the keto moiety of commercially available 3-chloro-1-phenylpropan-1-one was reduced with sodium borohydride and bromination of the resulting intermediate with aqueous hydrogen bromide led to the formation of 2 [10].
In the next reaction step, core compound 1 and side chain 2 were subjected to a condensation reaction under alkaline conditions (Scheme 1, (i)). Then, the chloro group of the resulting product was converted into an iodo group in a Finkelstein reaction (Scheme 1, (ii)). After purification, this intermediate was heated for 12 h in a solution of NaN3 and DMF to obtain the respective azide compound, which after treatment with triphenylphosphine eventually afforded target compound 3 (Scheme 1, (iii)).
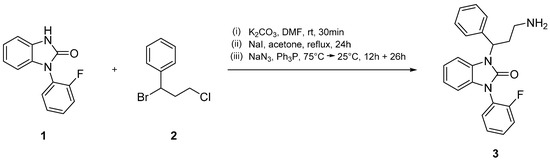
Scheme 1.
Reaction of 1-(2-fluorophenyl)-1,3-dihydro-2H-benzimidazol-2-one (1) and (1-bromo-3-chloropropyl)benzene (2) to 1-(3-amino-1-phenylpropyl)-3-(2-fluorophenyl)-1,3-dihydro-2H-benzimidazol-2-one (3).
3. Experimental Section
3.1. General Information
The NMR spectrum was recorded from a CDCl3 solution on a Bruker Avance III 400 spectrometer (Karlsruhe, Germany, 400 MHz for 1H, 100 MHz for 13C, 40 MHz for 15N, 376 MHz for 19F) at 25 °C. The center of the solvent (residual) signal was used as an internal standard which was related to trimethylsilane (TMS) with δ 7.26 ppm (1H in CDCl3), and δ 77.0 ppm (13C in CDCl3). 19F-NMR spectra were referenced by absolute referencing via Ξ ratio. Digital resolutions were 0.25 Hz/data point in the 1H and 0.3 Hz/data point in the 13C-NMR spectra. Coupling constants (J) are quoted in Hz. The following abbreviations were used to show the multiplicities: s: singlet, d: doublet, t: triplet, q: quadruplet, dd: doublet of doublet, m: multiplet. Mass spectra were obtained on a Shimadzu QP 1000 instrument (Kyoto, Japan, EI, 70 eV), high-resolution mass spectrometry (HRMS) was carried out on a maXis HD ESI-Qq-TOF mass spectrometer (Bruker Daltonics, Bremen, Germany) in the positive-ion mode by direct infusion. Compound purity: all compounds synthesized featured a purity of at least 95%.
3.2. 1-(3-Amino-1-phenylpropyl)-3-(2-fluorophenyl)-1,3-dihydro-2H-benzimidazol-2-one
- (i)
- To a solution of 1 (1.00 g, 4.38 mmol) in DMF (5 mL) was added K2CO3 (1.21 g, 8.76 mmol) and the resulting mixture was stirred for 30 min at 25 °C. After 30 min, 2 (1.53 g, 6.57 mmol) was added and stirring was continued overnight. Ethyl acetate (5 mL) and water (5 mL) were added to the mixture and the aqueous layer was extracted several times with ethyl acetate (10 mL). The combined organic layers were washed with brine, dried over MgSO4, and evaporated to dryness. Purification of the resulting product was carried out by column chromatography (silica gel 60) with petroleum ether/ethyl acetate 9:1 to afford an orange resin.
- (ii)
- The resulting orange resin (0.84 g, 2.20 mmol) was dissolved in acetone (7 mL) and NaI (0.66 g, 4.40 mmol) was added. The mixture was refluxed for 24 h, after which the formed precipitate was filtered and concentrated prior to purification via column chromatography (silica gel 60) with petroleum ether/ethyl acetate 9:1, to give the intermediate product as yellow crystals.
- (iii)
- The obtained product (0.15 g, 0.32 mmol) was dissolved in DMF (3 mL) and heated to 75 °C upon addition of NaN3 (0.05 g, 0.65 mmol). After 12 h the reaction was quenched with H2O (10 mL) and the resulting mixture was extracted with ethyl acetate (3 × 30 mL). The organic layer was washed with brine (3 × 10 mL), dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified via column chromatography (silica gel 60, petroleum ether/ethyl acetate 8.5:1.5) and was introduced in the subsequent reaction step.
Triphenylphosphine (0.07 g, 0.27 mmol) was added to a solution of the prior synthesized azide (0.07 g, 0.18 mmol) in THF (3 mL) and the mixture was stirred for 10 h at 25 °C. Thereafter, H2O was added and the reaction was stirred for another 16 h. The resulting reaction product was concentrated in vacuo and purified by column chromatography (silica gel 60, CH2Cl2/MeOH 9:1).
Yield: 0.04 g (57%), colorless crystals, m.p. 135–136 °C.
1H-NMR (400 MHz, CDCl3): δ (ppm) 2.44 (br s, 2H, NH2), 2.50–2.54 (m, 2H, 2′-CH2), 2.73–2.84 (m, 2H, 3′-CH2), 5.88–5.90 (m, 1H, 1′-CH), 6.82–6.84 (m, 2H, benzim 7-CH, benzim 4-CH), 6.93–7.02 (m, 2H, benzim 6-CH, benzim 5-CH), 7.26–7.37 (m, 5H, phen 4-CH, f-phen 3-CH, f-phen 5-CH, phen 3-CH, phen 5-CH), 7.42–7.57 (m, 4H, f-phen 6-CH, f-phen 4-CH, phen 2-CH, phen 6-CH).
13C-NMR (100 MHz, CDCl3): δ (ppm) 33.6 (2′-CH2), 38.6 (3′-CH2), 53.1 (1′-CH), 108.8 (d, J = 1.4 Hz, benzim 4-CH), 109.9 (benzim 7-CH), 117.1 (d, J = 19.5 Hz, f-phen 3-CH), 121.4 (benzim 5-CH), 121.9 (benzim 6-CH), 122.0 (f-phen 1-CH), 124.9 (d, J = 3.9 Hz, f-phen 5-CH), 127.2 (phen 2-CH), 127.2 (phen 6-CH), 127.7 (phen 4-CH), 128.1 (benzim 7a-C), 128.7 (phen 3-CH), 128.7 (phen 5-CH), 129.6 (f-phen 6-CH), 129.6 (benzim 3a-C), 130.2 (d, J = 7.8 Hz, f-phen 4-CH), 138.7 (phen 1-C), 153.5 (benzim 2-CO), 157.9 (d, J = 253.0 Hz, f-phen 2-CF).
19F-NMR (471 MHz, CDCl3): δ (ppm) −118.58 (m, f-phen CF), MS: m/z (%) 361 (M+, 17), 331 (10), 318 (11), 228 (100), 199 (21), 185 (18), 133 (20), 103 (17), 91 (32), 77 (25), 43 (25).
HRMS: m/z calculated for C22H21FN3O [M + H]+: 362.1663. Found: 362.1665.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Author Contributions
Catharina Neudorfer: Responsible for the performance of the syntheses, supervision and writing; Nadine Eberherr: Performance of the syntheses; Karem Shanab: Contributions to syntheses and experimental procedures; Wolfgang Holzer: Performance of the NMR analyses; Christina Rami-Mark: Designed parts of the research; Markus Mitterhauser: Designed parts of the research and proofread the manuscript; Wolfgang Wadsak: Designed parts of the research and proofread the manuscript; Helmut Spreitzer: Conceived and supervised the syntheses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, U.; Apparsundram, S.; Galli, A.; Kahlig, K.M.; Savchenko, V.; Schroeter, S.; Quick, M.W.; Blakely, R.D. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J. Neurosci. 2003, 23, 1697–1709. [Google Scholar] [PubMed]
- Kim, C.H.; Hahn, M.K.; Joung, Y.; Anderson, S.L.; Steele, A.H.; Mazei-Robinson, M.S.; Gizer, I.; Teicher, M.H.; Cohen, B.M.; Robertson, D.; et al. A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention-deficit hyperactivity disorder. Proc. Natl. Acad. Sci. USA 2006, 103, 19164–19169. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.K.; Robertson, D.; Blakely, R.D. A mutation in the human norepinephrine transporter gene (SLC6A2) associated with orthostatic intolerance disrupts surface expression of mutant and wild-type transporters. J. Neurosci. 2003, 23, 4470–4478. [Google Scholar] [PubMed]
- Mirbolooki, M.R.; Upadhyay, S.K.; Constantinescu, C.C.; Pan, M.L.; Mukherjee, J. Adrenergic pathway activation enhances brown adipose tissue metabolism: A [18F]FDG PET/CT study in mice. Nucl. Med. Biol. 2014, 41, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Fan, X.; Yeckel, C.W.; Weinzimmer, D.; Mulnix, T.; Gallezot, J.D.; Carson, R.E.; Sherwin, R.S.; Ding, Y.S. Ex vivo and in vivo Evaluation of the Norepinephrine Transporter Ligand [11C]MRB for Brown Adipose Tissue Imaging. Nucl. Med. Biol. 2012, 39, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Wadsak, W.; Mitterhauser, M. Basics and principles of radiopharmaceuticals for PET/CT. Eur. J. Radiol. 2010, 73, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Rami-Mark, C; Zhang, M.R.; Mitterhauser, M.; Lanzenberger, R.; Hacker, M.; Wadsak, W. [18F]FMeNER-D2: Reliable fully-automated synthesis for visualization of the norepinephrine transporter. Nucl. Med. Biol. 2013, 40, 1049–1054. [Google Scholar]
- Vanicek, T.; Spies, M.; Rami-Mark, C.; Savli, M.; Höflich, A.; Kranz, G.S.; Hahn, A.; Kutzelnigg, A.; Traub-Weidinger, T.; Mitterhauser, M.; et al. The norepinephrine transporter in attention-deficit/hyperactivity disorder investigated with positron emission tomography. JAMA Psychiatry 2014, 71, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Mark, C.; Bornatowicz, B.; Mitterhauser, M.; Hendl, M.; Nics, L.; Haeusler, D.; Lanzenberger, R.; Berger, M.L.; Spreitzer, H.; Wadsak, W. Development and automation of a novel NET-PET tracer: [C-11]Me@APPI. Nucl. Med. Biol. 2013, 40, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Neudorfer, C.; Seddik, A.; Shanab, K.; Jurik, A.; Rami-Mark, C.; Holzer, W.; Ecker, G.; Mitterhauser, M.; Wadsak, W.; Spreitzer, H. Synthesis and in Silico Evaluation of Novel Compounds for PET-Based Investigations of the Norepinephrine Transporter. Molecules 2015, 20, 1712–1730. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).