2,3,4,9-Tetrahydro-9-(3-hydroxy-1,4-dioxo-1H-dihydro-naphthalen-2-yl)-8-methoxy-3,3-dimethyl-1H-xanthen-1-one
Abstract
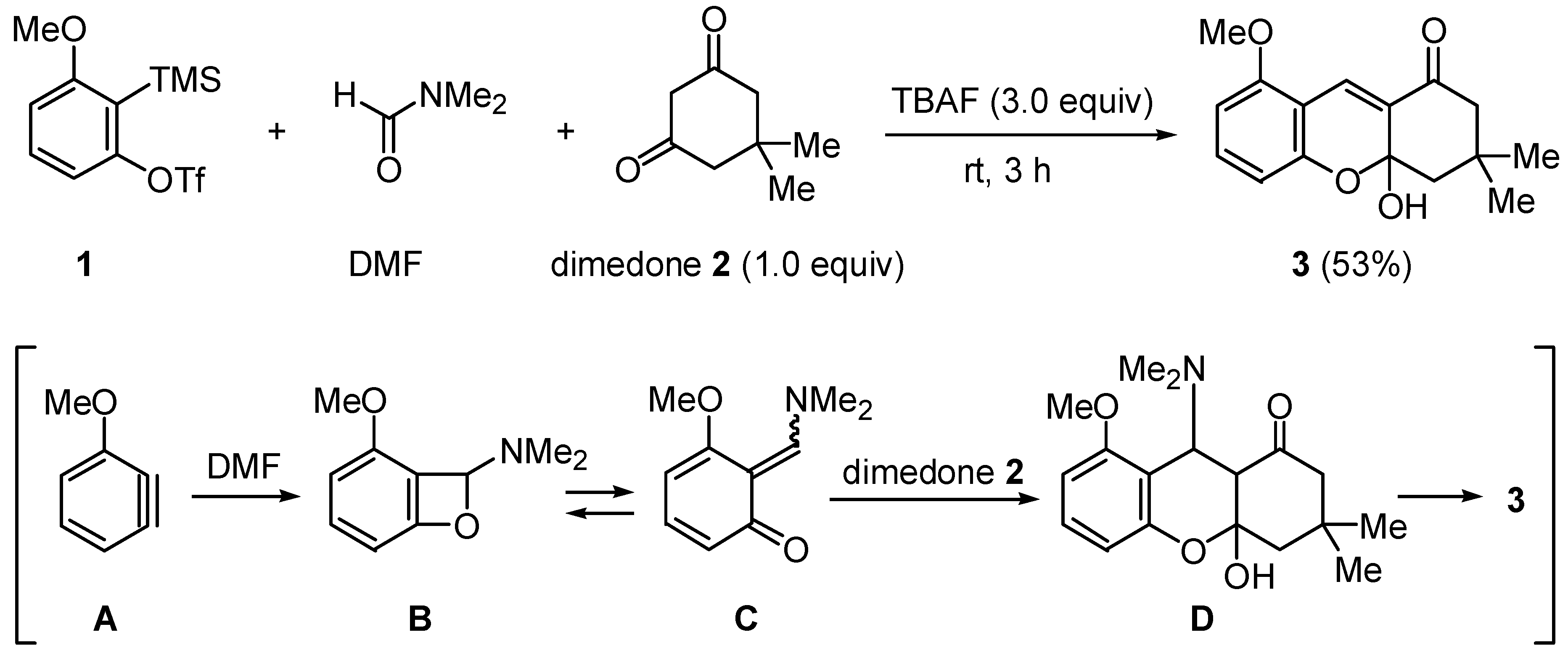
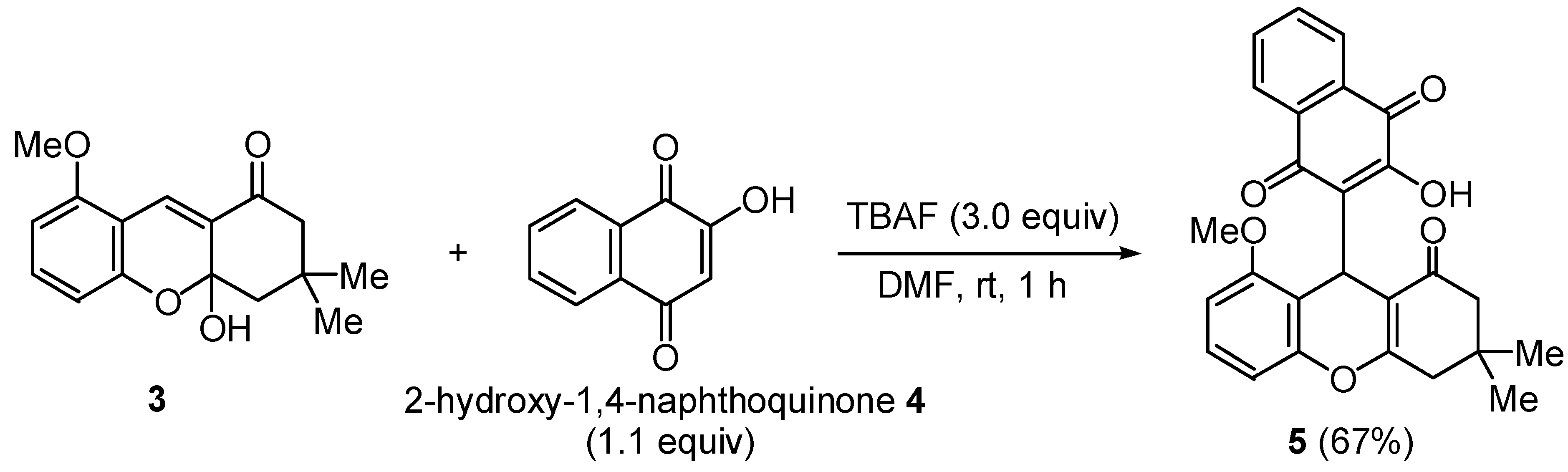
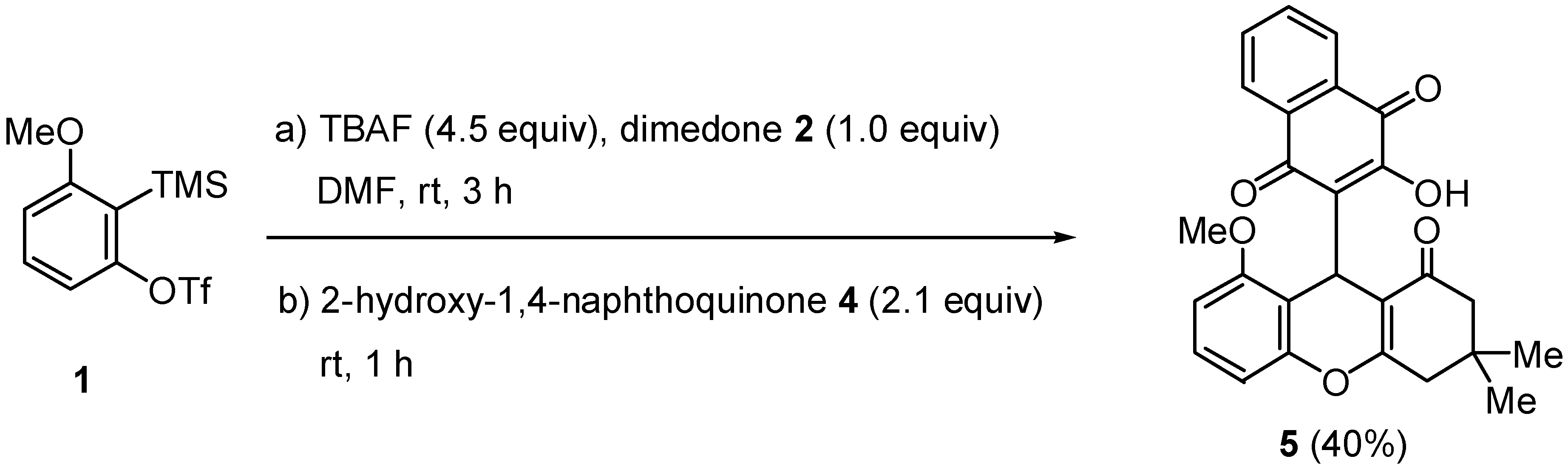
:Experimental
General Information
2,3,4,4a-Tetrahydro-4a-hydroxy-8-methoxy-3,3-dimethyl-1H-xanthen-1-one (3)
2,3,4,9-Tetrahydro-9-(3-hydroxy-1,4-dioxo-1H-dihydronaphthalen-2-yl)-8-methoxy-3,3-dimethyl-1H-xanthen-1-one (5)
Procedure for One-Pot Synthesis
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peña, D.; Pérez, D.; Guitián, E. Insertion of arynes into δ-bonds. Angew. Chem. Int. Ed. 2006, 45, 3579–3581. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Ohshita, J.; Kunai, A. Aryne, ortho-quinone methide, and ortho-quinodimethane: Synthesis of multisubstituted arenes using the aromatic reactive intermediates. Bull. Chem. Soc. Jpn. 2010, 83, 199–219. [Google Scholar] [CrossRef]
- Peña, D.; Escudero, S.; Pérez, D.; Guitián, E.; Castedo, L. Efficient palladium-catalyzed cyclotrimerization of arynes: Synthesis of triphenylenes. Angew. Chem. Int. Ed. 1998, 37, 2659–2661. [Google Scholar] [CrossRef]
- Yoshikawa, E.; Yamamoto, Y. Palladium-catalyzed intermolecular controlled insertion of benzyne-benzyne-alkene and benzyne-alkyne-alkene—Synthesis of phenanthrene and naphthalene derivatives. Angew. Chem. Int. Ed. 2000, 39, 173–175. [Google Scholar] [CrossRef]
- Liu, Z.; Larock, R.C. Palladium-catalyzed, sequential, three-component cross-coupling of aryl halides, alkynes, and arynes. Angew. Chem. Int. Ed. 2007, 46, 2535–2538. [Google Scholar] [CrossRef] [PubMed]
- Jayanth, T.T.; Cheng, C.-H. Nickel-catalyzed coupling of arynes, alkenes, and boronic acids: Dual role of the boronic acid. Angew. Chem. Int. Ed. 2007, 46, 5921–5924. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Shiotani, K.; Kinbara, A.; Sato, Y. Nickel-catalyzed [2+2+2] cycloaddition of arynes and an unactivated alkene: Synthesis of 9,10-dihydrophenanthrene derivatives. Chem. Commun. 2009, 4284–4286. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, L.; Zhao, Y.; Ni, C.; Zhao, J.; Hu, J. Silver-mediated trifluoromethylation-iodination of arynes. J. Am. Chem. Soc. 2013, 135, 2955–2958. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Fukushima, H.; Ohshita, J.; Kunai, A. CO2 incorporation reaction using arynes: Straightforward access to benzoxazinone. J. Am. Chem. Soc. 2006, 128, 11040–11041. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, C.D.; Allan, K.M.; Stoltz, B.M. Orthogonal synthesis of indolines and isoquinolines via aryne annulation. J. Am. Chem. Soc. 2008, 130, 1558–1559. [Google Scholar] [CrossRef] [PubMed]
- Sha, F.; Huang, X. A multicomponent reaction of arynes, isocyanides, and terminal alkynes: Highly chemo- and regioselective synthesis of polysubstituted pyridines and isoquinolines. Angew. Chem. Int. Ed. 2009, 48, 3458–3461. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Porwal, D.; Gonnade, R.G.; Biju, A.T. Multicomponent reactions involving arynes, quinolines, and aldehydes. Org. Lett. 2013, 15, 4620–4623. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Roy, T.; Pachfule, P.; Rajamohanan, P.R.; Biju, A.T. Transition-metal-free multicomponent reactions involving arynes, N-heterocycles, and isatins. Angew. Chem. Int. Ed. 2013, 52, 10040–10043. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.; Zhang, Y.; Cormier, M.; Chavez, G.; Arman, H.; Larionov, O.V. Three-component reaction of small-ring cyclic amine with arynes and acetonitrile. Chem. Comm. 2013, 49, 6558–6560. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Kohtani, S.; Miyabe, H. Sequential reaction of arynes via insertion into the π-bond of amides and trapping reaction with dialkylzincs. Org. Lett. 2010, 12, 1956–1959. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Miyabe, H. Insertion of arynes into the carbon-oxygen double bond of amides and its application into the sequential reactions. Tetrahedron 2012, 68, 179–189. [Google Scholar] [CrossRef]
- Yoshioka, E.; Kohtani, S.; Miyabe, H. A multicomponent coupling reaction induced by insertion of arynes into the C=O bond of formamide. Angew. Chem. Int. Ed. 2011, 50, 6638–6642. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Tanaka, H.; Kohtani, S.; Miyabe, H. Straightforward synthesis of dihydrobenzofurans and benzofurans from arynes. Org. Lett. 2013, 15, 3938–3941. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Kohtani, S.; Miyabe, H. Three-component coupling reactions of arynes for the synthesis of benzofurans and coumarins. Molecules 2014, 19, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Tamenaga, H.; Miyabe, H. [4+2] Cycloaddition of intermediates generated from arynes and DMF. Tetrahedron Lett. 2014, 55, 1402–1405. [Google Scholar] [CrossRef]
- Ramachary, D.B.; Jain, S. Sequential one-pot combination of multi-component and multi-catalysis cascade reactions: An emerging technology in organic synthesis. Org. Biomol. Chem. 2011, 9, 1277–1300. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Jitsuoka, M.; Shibata, T.; Hirohashi, T.; Nonoshita, K.; Moriya, M.; Haga, Y.; Sakuraba, A.; Ando, M.; Ohe, T.; et al. (9S)-9-(2-Hydroxy-4,4-dimethyl-6-oxo-1-cyclohexen-1-yl)-3,3-dimethyl-2,3,4,9-tetrahydro-1H-xanthen-1-one, a selective and orally active neuropeptide Y Y5 receptor antagonist. J. Med. Chem. 2008, 51, 4765–4770. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshioka, E.; Kohtani, S.; Miyabe, H. 2,3,4,9-Tetrahydro-9-(3-hydroxy-1,4-dioxo-1H-dihydro-naphthalen-2-yl)-8-methoxy-3,3-dimethyl-1H-xanthen-1-one. Molbank 2015, 2015, M841. https://doi.org/10.3390/M841
Yoshioka E, Kohtani S, Miyabe H. 2,3,4,9-Tetrahydro-9-(3-hydroxy-1,4-dioxo-1H-dihydro-naphthalen-2-yl)-8-methoxy-3,3-dimethyl-1H-xanthen-1-one. Molbank. 2015; 2015(1):M841. https://doi.org/10.3390/M841
Chicago/Turabian StyleYoshioka, Eito, Shigeru Kohtani, and Hideto Miyabe. 2015. "2,3,4,9-Tetrahydro-9-(3-hydroxy-1,4-dioxo-1H-dihydro-naphthalen-2-yl)-8-methoxy-3,3-dimethyl-1H-xanthen-1-one" Molbank 2015, no. 1: M841. https://doi.org/10.3390/M841
APA StyleYoshioka, E., Kohtani, S., & Miyabe, H. (2015). 2,3,4,9-Tetrahydro-9-(3-hydroxy-1,4-dioxo-1H-dihydro-naphthalen-2-yl)-8-methoxy-3,3-dimethyl-1H-xanthen-1-one. Molbank, 2015(1), M841. https://doi.org/10.3390/M841





