In the course of our work to prepare inhibitors of the enzyme dihydrofolate reductase, we desired to prepare sulfone analogues of some previously reported sulfides [1, 2]. The previously reported nitrosulfone [3] was not able to be reduced to the amine by using catalytic hydrogenation with a palladium on carbon catalyst, with tin and hydrochloric acid [4], or with iron and acetic acid in ethanol [5]. Therefore, the nitrosulfone, 1, was converted into the acetamide, 2, using iron in acetic acid [5]. The acetamide, 2, was hydrolyzed to the desired aniline, 3, using concentrated hydrochloric acid and ethanol. Unfortunately for us, the aniline was not able to be converted into the desired triazine by the reported procedure [2].
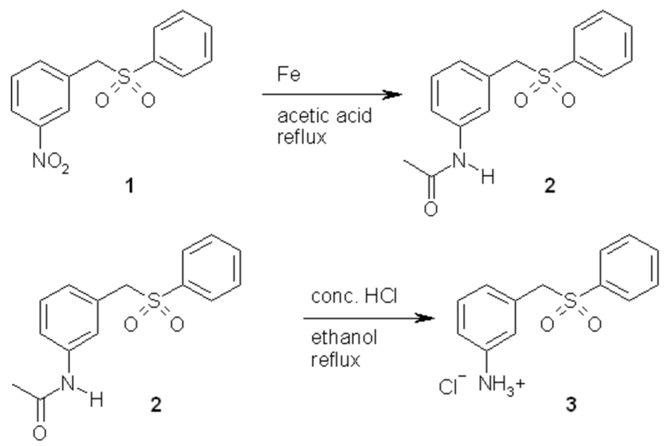
In round bottom flask 1-nitro-3-[(phenylsulfonyl)methyl]benzene, 1 (2.453 g, 8.85 mmoles), iron filings (2.019 g, 36.2 mmoles), and 25 mL of concentrated acetic acid were combined and refluxed overnight. After allowing the flask to cool, 200 mL of water and 100 mL of dichloromethane were added to the mixture. The unreacted iron was then removed by vacuum filtration. The aqueous and organic layers were separated, and the aqueous layer was extracted two more times with 45 mL portions of dichloromethane. The organic extracts were combined and dried with anhydrous magnesium sulfate. The dichloromethane was removed by using the rotavap. A yellow, viscous liquid was collected. After this liquid was allowed to stand for a couple of days, it solidified forming a tan solid, to yield 2.438 g (8.43 mmoles) of 3-[(phenylsulfonyl)methyl]acetanilide, 2. The percent yield for the reaction was 96%.
Melting Point: 121-123oC
IR (cm-1): 3280, 1661, 1593, 1300, 1161, 1152, 1083, 798, 777, 765, 698, 687.
1H-NMR (300 MHz, DMSO-d6): δ= 10.0 (1H, singlet), 7.7 (3H, multiplet), 7.6 (3H, multiplet), 7.5 (1H, singlet), 7.2 (1H, triplet, J = 7.7 Hz), 6.7 (1H, doublet, J = 7.7 Hz), 4.6 (2H, singlet), 2.0 (3H, singlet).
13C-NMR (75 MHz, DMSO-d6): δ= 168.3, 139.3, 138.4, 133.7, 129.1, 128.9, 128.4, 127.9, 125.6, 121.3, 118.9, 60.7, 23.9.
GC-MS [E.I., m/z (relative intensity)]: 148 (100), 106 (60), 77 (20), 77(16), 289 (M+, 14).
In a round bottom flask 3-[(phenylsulfonyl)methyl)acetanilide, 2 (1.003 g, 3.47 mmoles), 5 mL of 95% ethanol, and 6 mL of concentrated hydrochloric acid were combined and refluxed for 24 hours. The amide dissolved in the hot ethanol forming a yellow solution. After about 1 hour, a precipitate began to form. The reaction mixture was allowed to cool, and the precipitate was collected by vacuum filtration. The precipitate was washed with three portions of cold 95% ethanol to yield 0.893 g (3.15 mmoles) of 3-[(phenylsulfonyl)methyl]aniline hydrochloride, 3, was collected. The percent yield of the reaction was 91%.
Melting Point: 260 oC (decomposed)
IR (cm-1): 2879 (br), 1536, 1311, 1294, 1134, 1083, 765, 751, 720, 682.
1H-NMR (300 MHz, DMSO-d6): δ= 10.2 (3H, broad singlet), 7.7 (3H, multiplet), 7.6 (2H, multiplet), 7.3 (2H, multiplet), 7.2 (1H, singlet), 7.0 (1H, doublet, J = 7.1 Hz), 4.8 (2H, singlet).
13C-NMR (75 MHz, DMSO-d6): δ= 138.3, 133.9, 132.9, 130.3, 129.9, 129.5, 129.2, 127.9, 124.8, 122.7, 59.9.
GC-MS [E.I., m/z (relative intensity), free base]: 106 (100), 77 (26), 247 (M+, 18), 79 (16), 183 (12).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
The authors thank the Grants and Research Funding Committee of Southeast Missouri State University for financial support.
References:
- Selassie, C. D.; Guo, Z.-r.; Hansch, C.; Khwaja, T. A.; Pentacost, S. A Comparison of the Inhibition of Growth of Methotrexate-Resistant and -Sensitive Leukemia Cells in Culture by Triazines. Evidence of a New Mechanism of Cell Resistance to Methotrexate. J. Med. Chem. 1982, 25, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Hathaway, B. A.; Guo, Z.-r.; Selassie, C. D.; Dietrich, S. W.; Blaney, J. M.; Langridge, R.; Volz, K. W.; Kaufman, B. T. Crystallography, QSAR, and Molecular Graphics in a Comparative Analysis of the Inhibition of Dihydrofolate Reductase from Chicken Liver and L. casei by 4,6-Diamino-1,2-dihydro-2,2-dimethyl-1-(x-phenyl)-s-triazines. J. Med. Chem. 1984, 27, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, D. G; Hathaway, B. A. 1-Nitro-3-[(phenylsulfonyl)methyl]benzene. Molecules 2006, Mxxx. [Google Scholar] [CrossRef]
- Jones, A.G. The Selective Reduction of Meta-(and Para-) Nitroacetophenone. J. Chem. Ed. 1975, 10, 668–669. [Google Scholar] [CrossRef]
- Owsley, D. C.; Bloomfield, J. J. The Reduction of Nitroarenes with Iron/Acetic Acid. Synthesis. 1977, 118–120. [Google Scholar] [CrossRef]
© 2006 MDPI. All rights reserved.