As part of research programme directed to the synthesis of novel heterocyclic compounds pharmacologically interesting [1] via ring transformation of 1,2,4-triazine derivatives, we synthesized the title compounds 3 via two step process. Starting compounds, 5-(methoxyiminoethyl)-3-(methylsulfanyl)-1,2,4-triazine (1) [2] was easily oxidized under PTC-conditions into the corresponding sulfone 2. The latter compound, as reactive azadiene [3], was subjected in crude form to [4+2]cycloaddition/retro cycloaddition with 1-pyrrolidino-1-cyclohexene as dienophile to give 5,6,7,8-Tetrahydro-3-(1-methoxyiminoethyl)-1-methylsulfonylisoquinoline (3) in 83% yield.
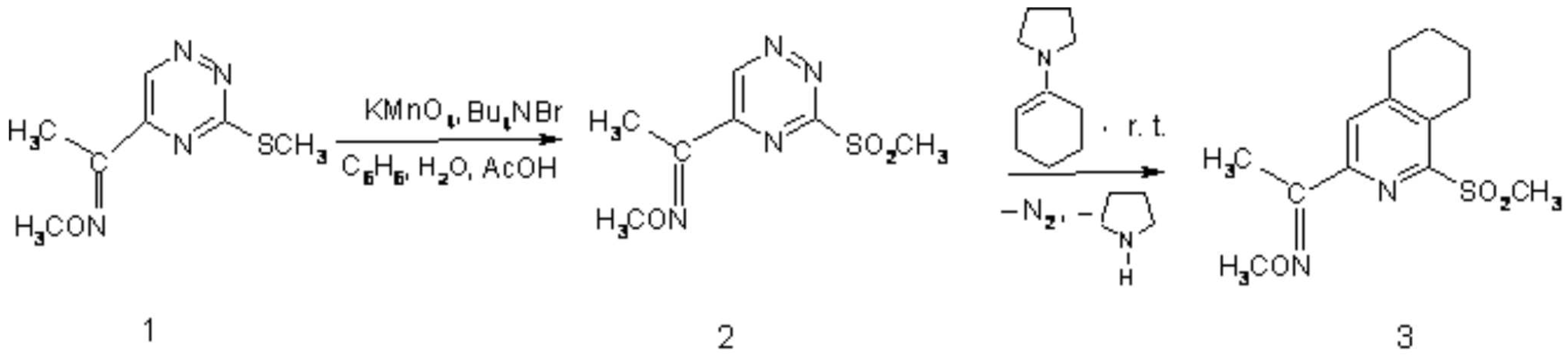
Preparation of 2:
A solution of KMnO4 (474 mg, 3 mmol) in water 20 ml was added to a solution of 1 (226 mg, 1 mmol) and Bu4N+Br- (48 mg, 1.5 mmol) in a mixture of AcOH (1.8 ml, 30 mmol) and benzene (15 ml) during stirring and cooling to 5-10oC. The reaction was continued at 10oC for 1-1.5 h, until complete oxidation process was observed with monitoring by TLC (CHCl3/acetone 50:1). A saturated solution of Na2S2O5 for decoloring, then saturated solution of K2CO3 for neutralization were added. The organic layer was separated and water phase was extracted with benzene (3 x 20 ml). The combined organic layers were washed with water (2 x 20 ml) and dried over MgSO4. After evaporation of the solvents under reduced pressure to volume of 5 ml, the solution was contained of pure product 2 (TLC monitoring) and was used for next step.
IR (KBr, ν, cm-1): 3025 (CHaromat.), 2970, 2850 (CH3); 1655 (C=N); 1555, 1465, 1395 (aromat. ring); 1340, 1180 (SO2); 1070 (C-O).
1H-NMR (CDCl3, 200 MHz): δ= 9.82 (s, 1 H, CHaromat.); 4.26 (s, 3 H, CH3O); 3.45 (s. 3H, CH3SO2); 2.43 (s, 3 H, H3C=N).
Preparation of 5,6,7,8-Tetrahydro-3-(1-methyoxyiminoethyl)-1-methylsulfonyl-isoquinoline (3):
To the solution of 2 (1 mmol) was added 1-(N-pyrrolidine)cyclohexene (302 mg, 2 mmol). Vigrously extrusion of N2 was observed. The reaction mixture was stirred for 5 h at room temperature, until the substrate 2 was disappeared (TLC monitoring: CHCl3/acetone-50:1). Removal of solvents under reduced pressure and purification of the residue by column chromatography on silica gel (230-400 mesh, Merck type 60) using chloroform as eluent gave 235 mg (83 %) of 5,6,7,8-tetrahydro-3-(1-methyoxyiminoethyl)-1-methylsulfonylisoquinoline (3) as a white solid after recrystalization from mixture chloroform/hexane 1:3.
Melting Point: 126-127oC
IR (KBr, ν, cm-1): 3030 (CHaromat.); 2975, 2875, 2790 (CH3 and CH2); 1660 (C=N); 1540, 1440 (aromat. ring); 1340, 1150 (SO2); 1060 (CH3O).
1H-NMR (CDCl3, 200 MHz): δ= 7.81 (s, 1 H, CHaromat.); 4.03 (s, 3 H, CH3O); 4.00 (s, 3 H, CH3SO2); 3.25 (t, J=5.5Hz, 2 H, C8H2); 2.85 (t, J=5.7 Hz, 2 H, C5H2); 2.24 (s, 3 H, CH3C=N); 1.94-1.76 (m, 4 H, C6H2C7H2).
MS (EI), m/z (% rel. int.): 282 (30) [M+.], 251 (15), 242 (11), 212 (13), 181(46), 170 (14), 170 (15), 151 (15), 149 (15), 137 (18), 135 (18), 125 (22), 123 (25), 111 (48), 109 (45), 97 (64), 95 (62), 85 (48), 83 (100), 81 (51), 71 (55), 69 (54).
HR-MS (EI): Calculated for C13H18N2O3S: 282.1038; Found: 282.1038.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References and notes:
- For previous paper in this series, see: Lipińska, T. Tetrahedron Lett. 2002, 43, 9565–9567.
- Lipińska, T.; Branowska, D.; Rykowski, A. Khim. Geterosikl. Soedin. 1999, 381–389.Chem. Heterocycl. Compd (Engl. Transl.) 2001, 37, 231–236.
- For a review on aza-DA-rDA reactions with extrusinon of small molecules see:Rickborn, B. Organic Reactions, Vol. 53, 224–627Paguette, Leo A., et al., Eds.; J. Wiley & Sons Inc., 1998.
- Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.