As part of research programme directed to the synthesis of novel heterocyclic compounds pharmacologically interesting [1] via ring transformation of 1,2,4-triazine derivatives, we synthesized the title compounds 3 via two step process. Starting compounds, 5-(methoxyiminobutyl)-3-(methylsulfanyl)-1,2,4-triazine (1) [2] was easily oxidized under PTC-conditions into the corresponding sulfone 2. The latter compound, as reactive azadiene [3], was subjected in crude form to [4+2]cycloaddition-retro cycloaddition with 1-pyrrolidino-1-cyclohexane as dienophile to give 5,6,7,8-Tetrahydro-3-(1-methoxyiminoethyl)-1-methylsulfonylisoquinoline (3) in 70% yield.
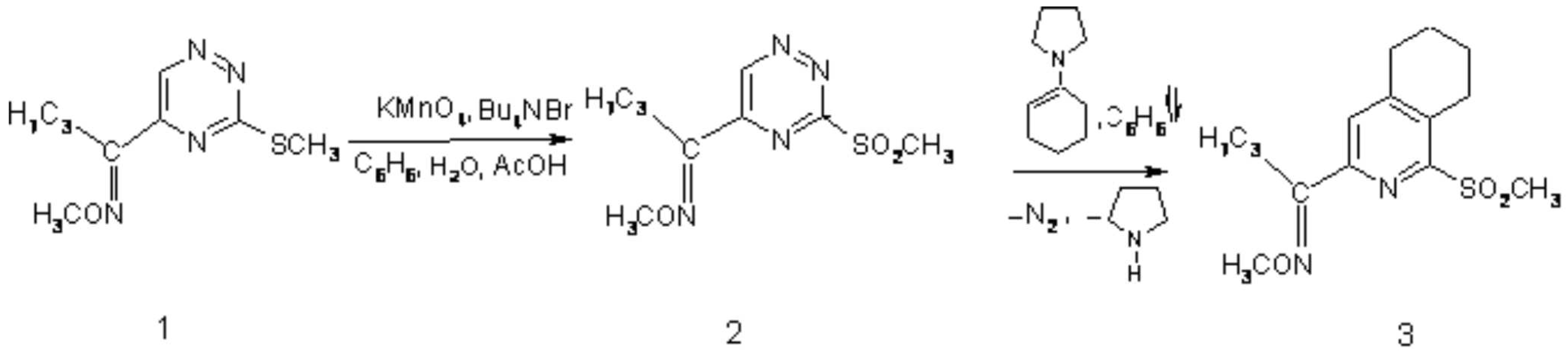
Preparation of 2:
A solution of KMnO4 (474 mg, 3 mmol) in water 20 ml) was added to a solution of 1 (226 mg, 1 mmol) and Bu4N+Br- (48 mg, 1.5 mmol) in a mixture of AcOH (1.8 ml, 30 mmol) and benzene (15 ml) during stirring and cooling to 5-10oC. The reaction was continued at 10oC for 1-1.5 h, until complete oxidation process was observed with monitoring by TLC (CHCl3/acetone -50:1). A saturated solution of Na2S2O5 for decoloring, then saturated solution of K2CO3 for neutralization were added. The organic layer was separated and water phase was extracted with benzene (3 x 20 ml).
The combined organic layers were washed with water (2 x 20 ml) and dried over MgSO4. After evaporation of the solvents under reduced pressure to volume of 5 ml, the solution was contained of pure product 2 (TLC monitoring) and was used for next step.
IR (KBr, ν, cm-1): 3030 (CHaromat.); 2975, 2850 (CH3); 1665 (C=N); 1565, 1460, 1395 (aromat. ring); 1335, 1155 (SO2); 1075 (C-O);
1H-NMR (CDCl3, 200 MHz); δ= 9.64 (s, 1 H, CHaromat.); 4.20 (s, 3 H, CH3O); 3.45 (s, 3H, CH3SO2); 2.90 (t, J= 7.7 Hz, 2 H, H2CC=N); 1.60 (m, 2 H, H2CCH2C=); 1.05 (t, J=7.4 Hz, 3 H, H3CCH2).
Preparation of 5,6,7,8-Tetrahydro-3-(1-methoxyiminobutyl)-1-methylsulfonylisoquinoline (3):
To the freshly obtained solution of 2 (1 mmol), was added 1-(N-pyrrolidine)cyclohexene (302 mg, 2 mmol). The reaction mixture was refluxed for 1h until the substrate 2 was disappeared (TLC monitoring: CHCl3/acetone-50:1). Removal of solvents under reduced pressure and purification of the residue by column chromatography on silica gel (230-400 mesh, Merck type 60) using chloroform/hexane - 3:1 as eluent gave 218 mg (70 %) of 5,6,7,8-tetrahydro-3-(1-methyoxyiminobutyl)-1-methylsulfonylisoquinoline (3) as a white solid after recrystalization from mixture chloroforme/hexane 1:2.
Melting point: 84-85oC
IR (KBr, ν, cm-1): 3030 (CHaromat.); 2975, 2875, 2855 (CH3, CH2); 1660 (C=N); 1550, 1460 (aromat. ring); 1340, 1170 (SO2); 1070 (CH3O).
1H-NMR (CDCl3, 200 MHz): δ= 7.71 (s, 1 H, CHaromat.); 3.95 (s, 3 H, CH3O); 3.70 (s, 3 H, CH3SO2); 3.16 (t, J=5.5Hz, 2 H, C8H2); 2.83 (t, J=5.7 Hz, 2 H, C5H2); 2.64 (t, J=7.6 Hz, 2 H, CH2C=N); 1.94-1.76 (m, 4 H, C6H2C7H2); 1.61 (m, 2 H, H2CCH3); 0.97 (t, J=7.5 Hz, 3H, H3CCH2).
MS (EI), m/z (% rel. int.): 310 (100) [M+]; 295 (13); 279 (35); 267 (13); 250 (15); 212 (25); 181 (33).
Elemental Analysis: Calculated for C15H22N2O3S: C, 58.04%; H, 7.14%; N, 9.03%. Found: C, 57.88%; H, 7.20%; N, 8.90%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References and notes:
- For previous paper in this series, see: Lipińska, T. Tetrahedron Lett. 2002, 43, 9565–9567.
- Lipińska, T.; Branowska, D.; Rykowski, A. Khim. Geterosikl. Soedin. 1999, 381–389.Chem. Heterocycl. Compd (Engl. Transl.) 2001, 37, 231–236.
- For a review on aza-DA-rDA reactions with extrusinon of small molecules see:Rickborn, B. Organic Reactions, Vol. 53, 224–627Paguette, Leo A., et al., Eds.; J. Wiley & Sons Inc., 1998.
- Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.