Association of Galectin-9 Soluble Immune Checkpoint with Clinical Prognostic Markers in Patients with Chronic Lymphocytic Leukemia
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics and Hematological Parameters
2.2. Clinical Staging and Prognostic Markers in Patients with CLL
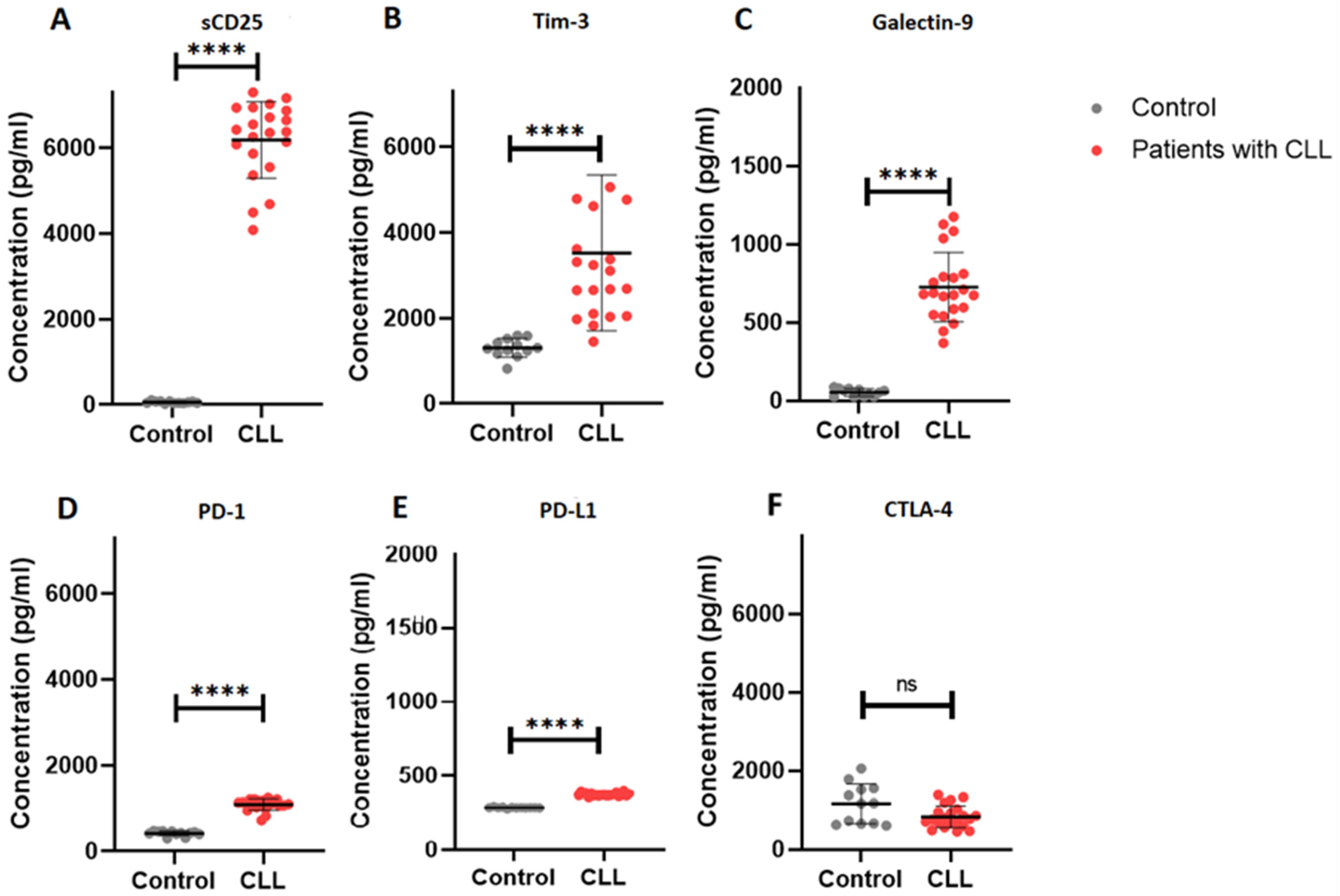
2.3. Elevated Soluble Immune Checkpoint Levels in Patients with CLL
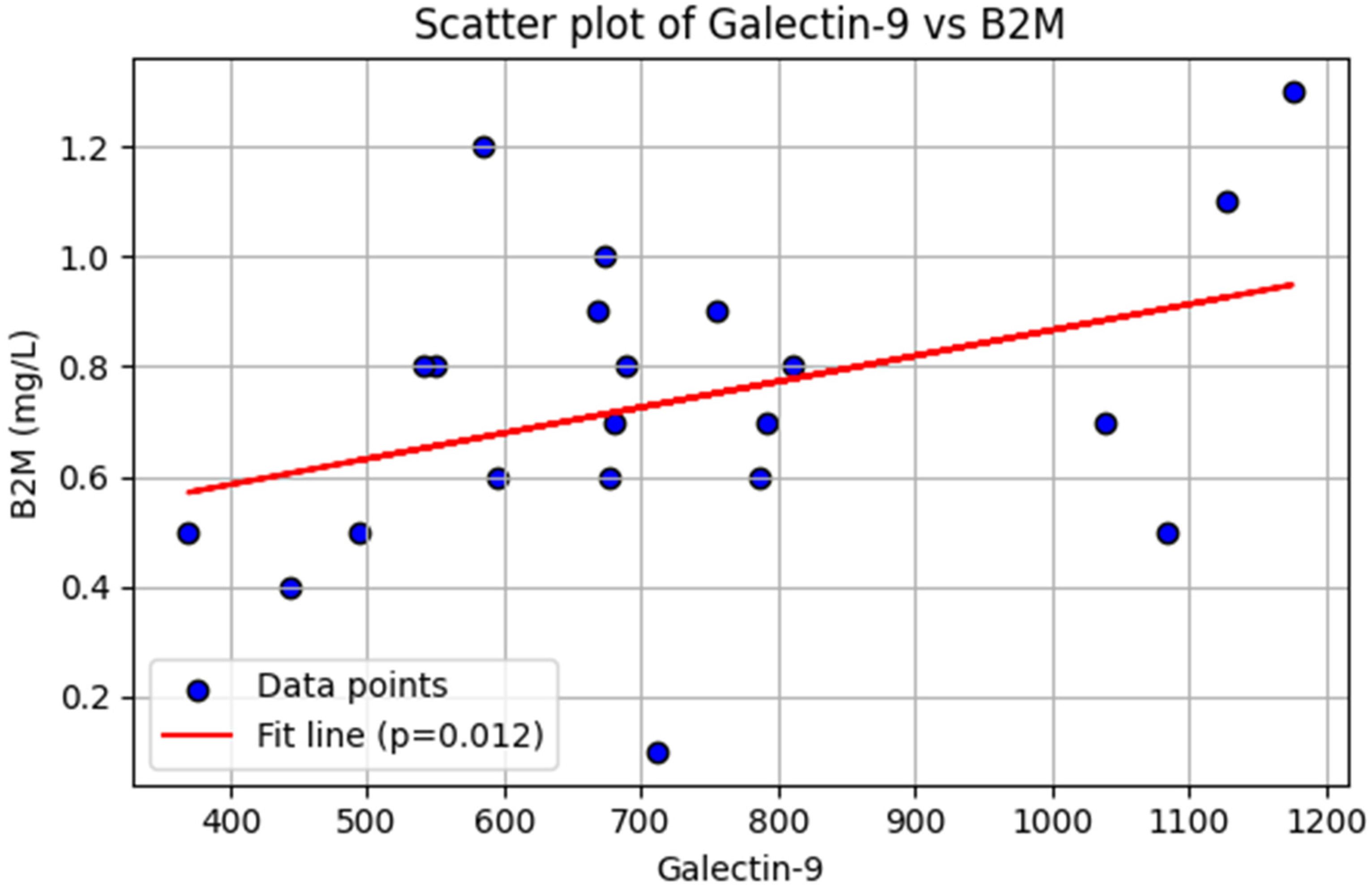
2.4. Association of Galectin-9 with β2 Microglobulin in Patients with CLL
3. Discussion
4. Methods and Materials
4.1. Patients Recruitment
4.2. Inclusion and Exclusion Criteria
4.3. Sample Size Estimation
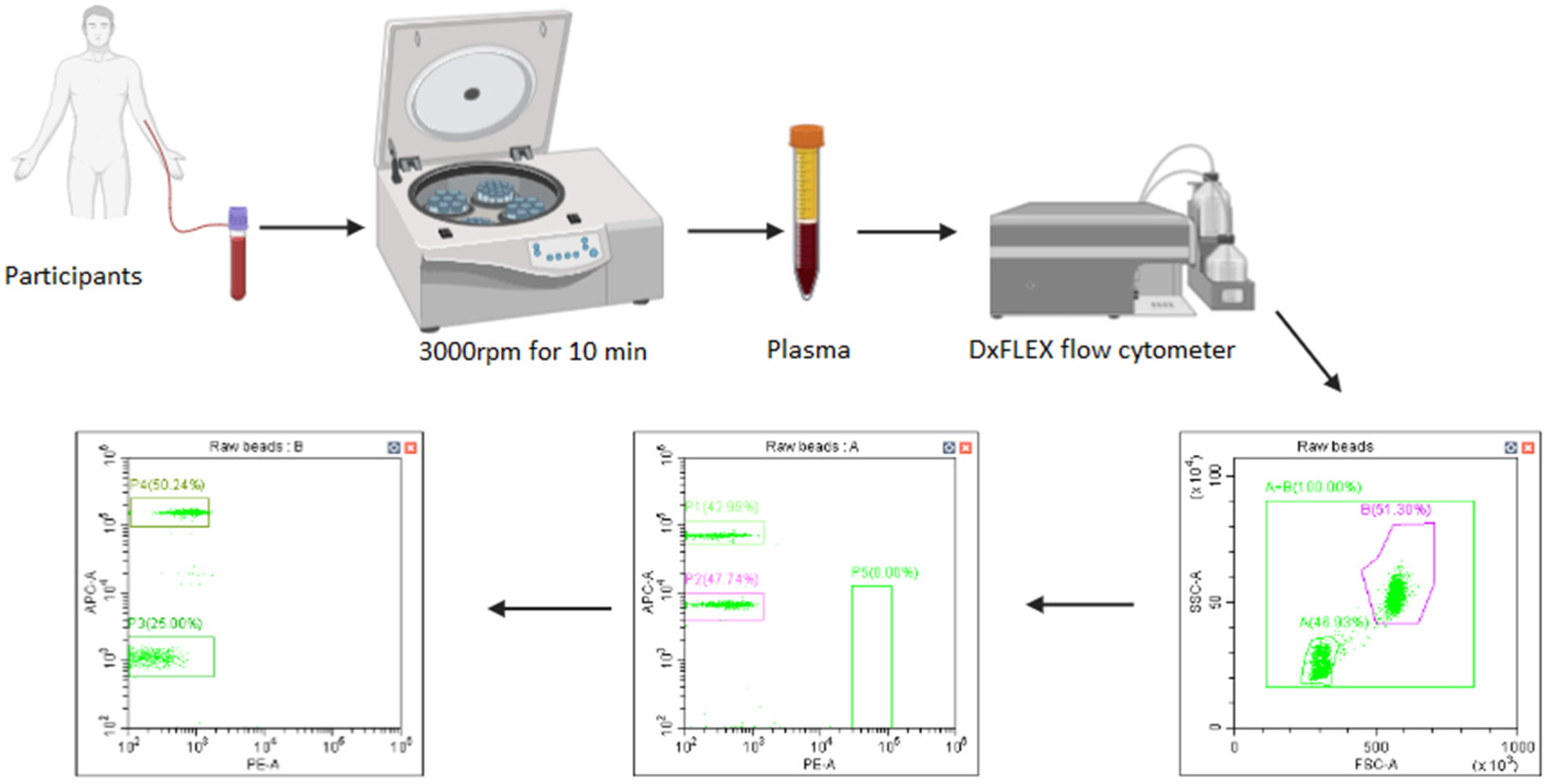
4.4. Sample Collection
4.5. Measurement of Soluble Immune Checkpoint Profiles
4.6. Measurements of Serum Soluble Beta-2-Microglobulin (B2M) Levels
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trivedi, P.J.; Patel, D.M.; Kazi, M.; Varma, P. Cytogenetic Heterogeneity in Chronic Lymphocytic Leukemia. J. Assoc. Genet. Technol. 2023, 49, 4–9. [Google Scholar]
- Burger, J.A. Treatment of chronic lymphocytic leukemia. N. Engl. J. Med. 2020, 383, 460–473. [Google Scholar] [CrossRef]
- Braish, J.; Cerchione, C.; Ferrajoli, A. An overview of prognostic markers in patients with CLL. Front. Oncol. 2024, 14, 1371057. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; Stamatopoulos, K.; Belessi, C.; Moreno, C.; Stilgenbauer, S.; Stevenson, F.; Davi, F.; Rosenquist, R. ERIC recommendations on IGHV gene mutational status analysis in chronic lymphocytic leukemia. Leukemia 2007, 21, 1–3. [Google Scholar] [CrossRef]
- Thompson, P.A.; Bazinet, A.; Wierda, W.G.; Tam, C.S.; O’Brien, S.M.; Saha, S.; Peterson, C.B.; Plunkett, W.; Keating, M.J. Sustained remissions in CLL after frontline FCR treatment with very-long-term follow-up. Blood 2023, 142, 1784–1788. [Google Scholar] [CrossRef]
- Vu, M.; Degeling, K.; Thompson, E.R.; Blombery, P.; Westerman, D.; IJzerman, M.J. Cost Effectiveness of Molecular Diagnostic Testing Algorithms for the Treatment Selection of Frontline Ibrutinib for Patients with Chronic Lymphocytic Leukemia in Australia. Appl. Health Econ. Health Policy 2024, 22, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, C.; Raca, G.; Wang, Y.L. Predicting prognosis in chronic lymphocytic leukemia in the contemporary era. JAMA Oncol. 2015, 1, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.A.; Strati, P.; Tsang, M.; West, C.P.; Shanafelt, T.D. Should IGHV status and FISH testing be performed in all CLL patients at diagnosis? A systematic review and meta-analysis. Blood J. Am. Soc. Hematol. 2016, 127, 1752–1760. [Google Scholar] [CrossRef]
- Döhner, H.; Stilgenbauer, S.; Benner, A.; Leupolt, E.; Kröber, A.; Bullinger, L.; Döhner, K.; Bentz, M.; Lichter, P. Genomic aberrations and survival in chronic lymphocytic leukemia. N. Engl. J. Med. 2000, 343, 1910–1916. [Google Scholar] [CrossRef]
- Hallek, M.; Fischer, K.; Fingerle-Rowson, G.; Fink, A.M.; Busch, R.; Mayer, J.; Hensel, M.; Hopfinger, G.; Hess, G.; Von Grünhagen, U. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet 2010, 376, 1164–1174. [Google Scholar] [CrossRef]
- Chavez, J.C.; Kharfan-Dabaja, M.A.; Kim, J.; Yue, B.; Dalia, S.; Pinilla-Ibarz, J.; Anasetti, C.; Locke, F.L. Genomic aberrations deletion 11q and deletion 17p independently predict for worse progression-free and overall survival after allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia. Leuk. Res. 2014, 38, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Burger, J.A.; Blum, K.A.; Coleman, M.; Wierda, W.G.; Jones, J.A.; Zhao, W.; Heerema, N.A. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood J. Am. Soc. Hematol. 2015, 125, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Varghese, A.M.; Sood, N.; Chiattone, C.; Akinola, N.O.; Huang, X.; Gale, R.P. Ethnic and geographic diversity of chronic lymphocytic leukaemia. Leukemia 2021, 35, 433–439. [Google Scholar] [CrossRef]
- Wdowiak, K.; Gallego-Colon, E.; Francuz, T.; Czajka-Francuz, P.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wybraniec, M.T.; Chudek, J.; Wojnar, J. Increased serum levels of Galectin-9 in patients with chronic lymphocytic leukemia. Oncol. Lett. 2019, 17, 1019–1029. [Google Scholar] [CrossRef]
- Alimu, X.; Zhang, J.; Pang, N.; Zhang, R.; Chen, R.; Zeng, X.; Tudahong, S.; Chen, G.; Muhashi, M.; Zhao, F. Galectin-9 and myeloid-derived suppressor cell as prognostic indicators for chronic lymphocytic leukemia. Immun. Inflamm. Dis. 2023, 11, e853. [Google Scholar] [CrossRef]
- Pang, N.; Alimu, X.; Chen, R.; Muhashi, M.; Ma, J.; Chen, G.; Zhao, F.; Wang, L.; Qu, J.; Ding, J. Activated Galectin-9/Tim3 promotes Treg and suppresses Th1 effector function in chronic lymphocytic leukemia. FASEB J. 2021, 35, e21556, Correction in FASEB J. 2024, 38, e23740. https://doi.org/10.1096/fj.202401212. [Google Scholar] [CrossRef]
- Yi, J.S.; Cox, M.A.; Zajac, A.J. T-cell exhaustion: Characteristics, causes and conversion. Immunology 2010, 129, 474–481. [Google Scholar] [CrossRef]
- Grzywnowicz, M.; Karczmarczyk, A.; Skorka, K.; Zajac, M.; Zaleska, J.; Chocholska, S.; Tomczak, W.; Giannopoulos, K. Expression of programmed death 1 ligand in different compartments of chronic lymphocytic leukemia. Acta Haematol. 2015, 134, 255–262. [Google Scholar] [CrossRef]
- Gamaleldin, M.; Ghallab, O.; Nadwan, E.; Abo Elwafa, R. PD-1 and PD-L1 gene expressions and their association with Epstein-Barr virus infection in chronic lymphocytic leukemia. Clin. Transl. Oncol. 2021, 23, 2309–2322. [Google Scholar] [CrossRef] [PubMed]
- Ciszak, L.; Frydecka, I.; Wolowiec, D.; Szteblich, A.; Kosmaczewska, A. CTLA-4 affects expression of key cell cycle regulators of G0/G1 phase in neoplastic lymphocytes from patients with chronic lymphocytic leukaemia. Clin. Exp. Med. 2016, 16, 317–332. [Google Scholar] [CrossRef]
- Fidan, K. Chronic lymphocytic leukemia. J. Curr. Hematol. Oncol. Res. 2023, 1, 59–67. [Google Scholar] [CrossRef]
- Ntsethe, A.; Mkhwanazi, Z.A.; Dludla, P.V.; Nkambule, B.B. B Cell Subsets and Immune Checkpoint Expression in Patients with Chronic Lymphocytic Leukemia. Curr. Issues Mol. Biol. 2024, 46, 1731–1740. [Google Scholar] [CrossRef]
- Ntsethe, A.; Dludla, P.V.; Nkambule, B.B. Impact of Protein Kinase C Activation and Monoclonal Antibodies on Immune Checkpoint Regulation and B Cell Function in Patients with Chronic Lymphocytic Leukemia. Biomedicines 2025, 13, 741. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.R.; Sawitsky, A.; Cronkite, E.P.; Chanana, A.D.; Levy, R.N.; Pasternack, B.S. Clinical staging of chronic lymphocytic leukemia. Blood 1975, 46, 219–234. [Google Scholar] [CrossRef]
- Xierenguli, A.; Zeng, X.; Pang, N.; Zhagn, R.; Ma, J.; Zhao, Y.; Qu, J. TIM-3/galectin-9 is involved in negative regulation of T cells in patients with chronic lymphocytic leukemia. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2020, 36, 1021–1025. [Google Scholar]
- Du, W.; Yang, M.; Turner, A.; Xu, C.; Ferris, R.L.; Huang, J.; Kane, L.P.; Lu, B. TIM-3 as a Target for Cancer Immunotherapy and Mechanisms of Action. Int. J. Mol. Sci. 2017, 18, 645. [Google Scholar] [CrossRef]
- Sheng, C.C.; Han, F.Y. Immunoregulation effects of TIM-3 on tumors. Neoplasma 2019, 66, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Taghiloo, S.; Allahmoradi, E.; Ebadi, R.; Tehrani, M.; Hosseini-Khah, Z.; Janbabaei, G.; Shekarriz, R.; Asgarian-Omran, H. Upregulation of Galectin-9 and PD-L1 immune checkpoints molecules in patients with chronic lymphocytic leukemia. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 2269. [Google Scholar] [PubMed]
- Wang, J.; Li, C.; Fu, J.; Wang, X.; Feng, X.; Pan, X. Tim-3 regulates inflammatory cytokine expression and Th17 cell response induced by monocytes from patients with chronic hepatitis B. Scand. J. Immunol. 2019, 89, e12755. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Nafady, A.; Ahmed, E.H.; Hassan, E.E.N.; Soliman, W.G.M.; Elbadry, M.I.; Allam, A.A. CXC chemokine ligand 13 and galectin-9 plasma levels collaboratively provide prediction of disease activity and progression-free survival in chronic lymphocytic leukemia. Ann. Hematol. 2024, 103, 781–792. [Google Scholar] [CrossRef]
- Nelson, B.H.; Willerford, D.M. Biology of the interleukin-2 receptor. Adv. Immunol. 1998, 70, 1–81. [Google Scholar] [PubMed]
- Sulda, M.L.; Kuss, B.J.; Hall, R.K.; Bailey, S.; Macardle, P.J. Clinical utility of molecular and flow cytometric markers in chronic lymphocytic leukaemia. Intern. Med. J. 2012, 42, 137–146. [Google Scholar] [CrossRef]
- Shvidel, L.; Braester, A.; Bairey, O.; Rahimi-Levene, N.; Herishanu, Y.; Tadmor, T.; Klepfish, A.; Ruchlemer, R.; Shtalrid, M.; Berrebi, A.; et al. Cell surface expression of CD25 antigen (surface IL-2 receptor α-chain) is not a prognostic marker in chronic lymphocytic leukemia: Results of a retrospective study of 281 patients. Ann. Hematol. 2012, 91, 1597–1602. [Google Scholar] [CrossRef]
- Brusa, D.; Serra, S.; Coscia, M.; Rossi, D.; D’Arena, G.; Laurenti, L.; Jaksic, O.; Fedele, G.; Inghirami, G.; Gaidano, G. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica 2013, 98, 953–963. [Google Scholar] [CrossRef]
- Ding, W.; LaPlant, B.R.; Call, T.G.; Parikh, S.A.; Leis, J.F.; He, R.; Shanafelt, T.D.; Sinha, S.; Le-Rademacher, J.; Feldman, A.L. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood J. Am. Soc. Hematol. 2017, 129, 3419–3427. [Google Scholar] [CrossRef]
- Knauf, W.; Langenmayer, I.; Ehlers, B.; Mohr, B.; Adorf, D.; Nerl, C.; Hallek, M.; Zwingers, T.; Emmerich, B.; Thiel, E. Serum levels of soluble CD23, but not soluble CD25, predict disease progression in early stage B-cell chronic lymphocytic leukemia. Leuk. Lymphoma 1997, 27, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Takács, F.; Tolnai-Kriston, C.; Hernádfői, M.; Szabó, O.; Szalóki, G.; Szepesi, Á.; Czeti, Á.; Matolcsy, A.; Barna, G. The Effect of CD86 Expression on the Proliferation and the Survival of CLL Cells. Pathol. Oncol. Res. 2019, 25, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Barna, G.; Szalóki, G.; Márk, Á.; Hunyadi, A.; Kriston, C. CD86, the double agent: Significance of CD86 expression in B-cell malignancies. Int. J. Cancer 2025, 157, 1772–1780. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood J. Am. Soc. Hematol. 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- STATA18. Available online: http://www.stata.com/ (accessed on 15 June 2024).
| Control (n = 12) | Patients with CLL [21] | p-Value | |
|---|---|---|---|
| Gender | |||
| Male, n (%) | 7 (58.33) | 13 (61.9) | |
| Female, n (%) | 5 (41.67) | 8 (38.1) | |
| Age (Years) | 56.58 ± 15.67 | 62.33 ± 13.31 | 0.2714 |
| Hematological parameters | |||
| White blood cell count (103 µL) | 5.26 ± 1.38 | 130.4 ± 29.71 | 0.0005 |
| Red blood cell (106 µL) | 4.74 ± 0.94 | 2.10 ± 0.84 | <0.0001 |
| Hemoglobin (g/dL) | 14.13 ± 3.81 | 8.19 ± 2.30 | <0.0001 |
| Platelets (103 µL) | 210.4 ± 73.14 | 157.5 ± 141.9 | 0.1831 |
| CD38% positive B cells | 28.47 ± 19.01 | 57.39 ± 8.001 | 0.0002 |
| Clinical Parameters | |
|---|---|
| RAI Staging | |
| I, n (%) | 0 (0) |
| II, n (%) | 5 (23.8) |
| III, n (%) | 6 (28.6) |
| IV, n (%) | 10 (47.6) |
| FISH Status | |
| Trisomy 12, n (%) | 1 (4.8) |
| Deletions | |
| 11q22, n (%) | 7 (33.3) |
| 13q14, n (%) | 6 (28.6) |
| 17p13, n (%) | 3 (14.3) |
| no abnormalities, n (%) | 4 (19.0) |
| CLL-IPI | |
| Low risk, n (%) | 14 (66.7) |
| Intermediate risk, n (%) | 4 (19) |
| High risk, n (%) | 3 (14.3) |
| Prognostic Biomarkers | |
| B2M mg/L | 0.74 ± 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ntsethe, A.; Dludla, P.V.; Nkambule, B.B. Association of Galectin-9 Soluble Immune Checkpoint with Clinical Prognostic Markers in Patients with Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2026, 27, 98. https://doi.org/10.3390/ijms27010098
Ntsethe A, Dludla PV, Nkambule BB. Association of Galectin-9 Soluble Immune Checkpoint with Clinical Prognostic Markers in Patients with Chronic Lymphocytic Leukemia. International Journal of Molecular Sciences. 2026; 27(1):98. https://doi.org/10.3390/ijms27010098
Chicago/Turabian StyleNtsethe, Aviwe, Phiwayinkosi Vusi Dludla, and Bongani Brian Nkambule. 2026. "Association of Galectin-9 Soluble Immune Checkpoint with Clinical Prognostic Markers in Patients with Chronic Lymphocytic Leukemia" International Journal of Molecular Sciences 27, no. 1: 98. https://doi.org/10.3390/ijms27010098
APA StyleNtsethe, A., Dludla, P. V., & Nkambule, B. B. (2026). Association of Galectin-9 Soluble Immune Checkpoint with Clinical Prognostic Markers in Patients with Chronic Lymphocytic Leukemia. International Journal of Molecular Sciences, 27(1), 98. https://doi.org/10.3390/ijms27010098







