Vesicular Transport Mediated by Endoplasmic Reticulum Stress Sensor BBF2H7 Orchestrates Melanin Production During Melanogenesis
Abstract
1. Introduction
2. Results
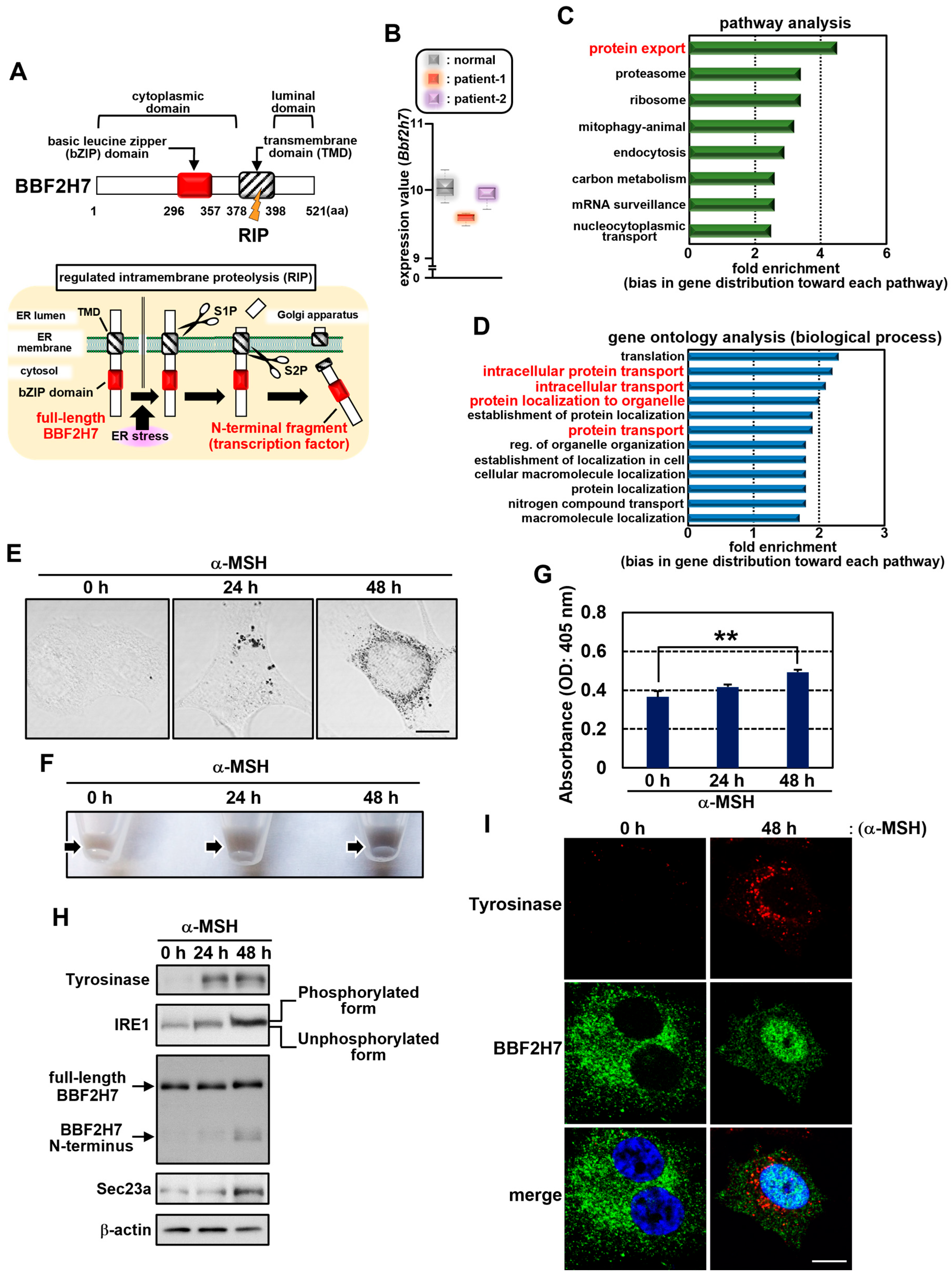
2.1. BBF2H7 Is Activated During Melanogenesis
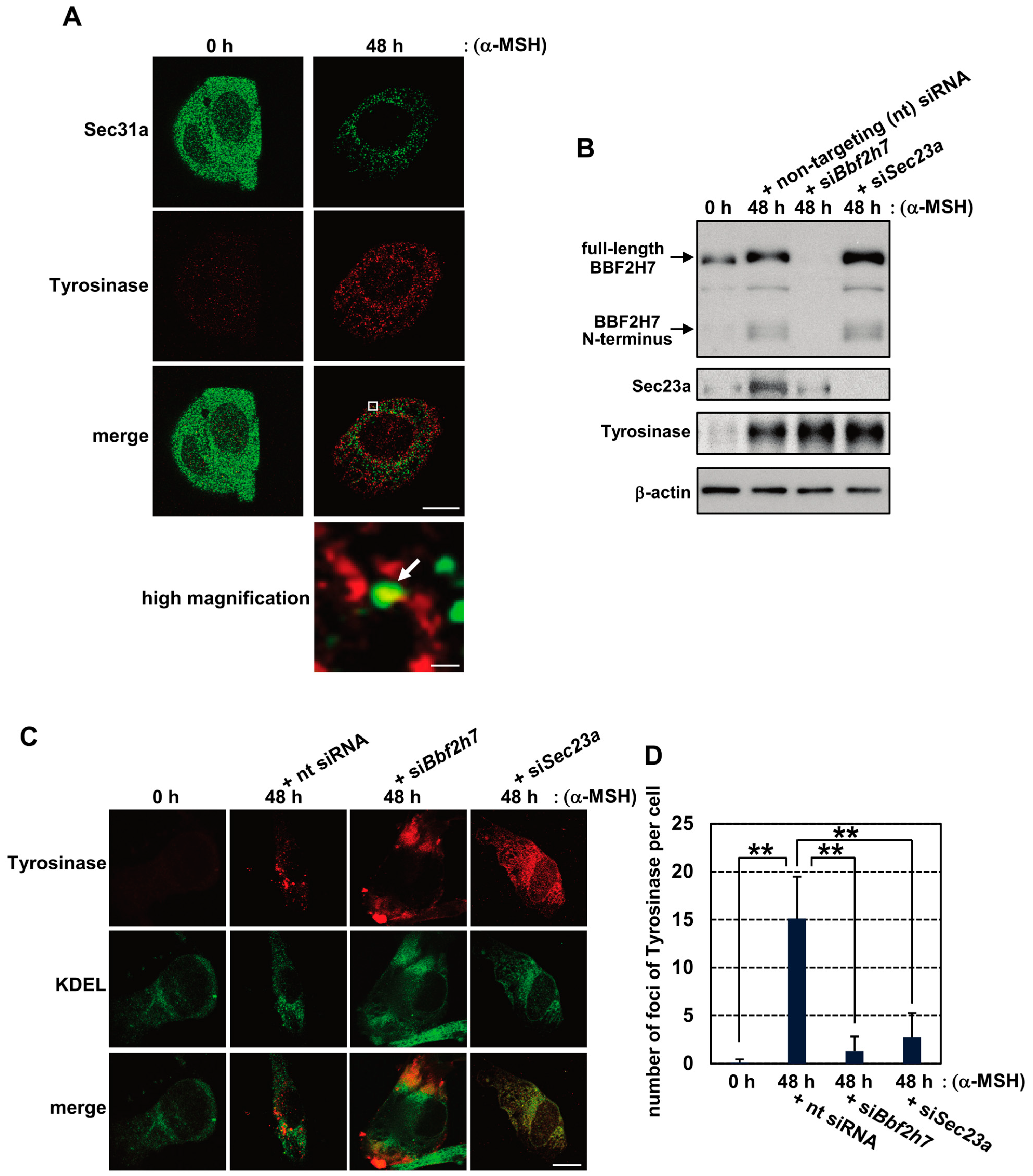
2.2. Activation of BBF2H7 Is Necessary for Vesicular Transport of Tyrosinase
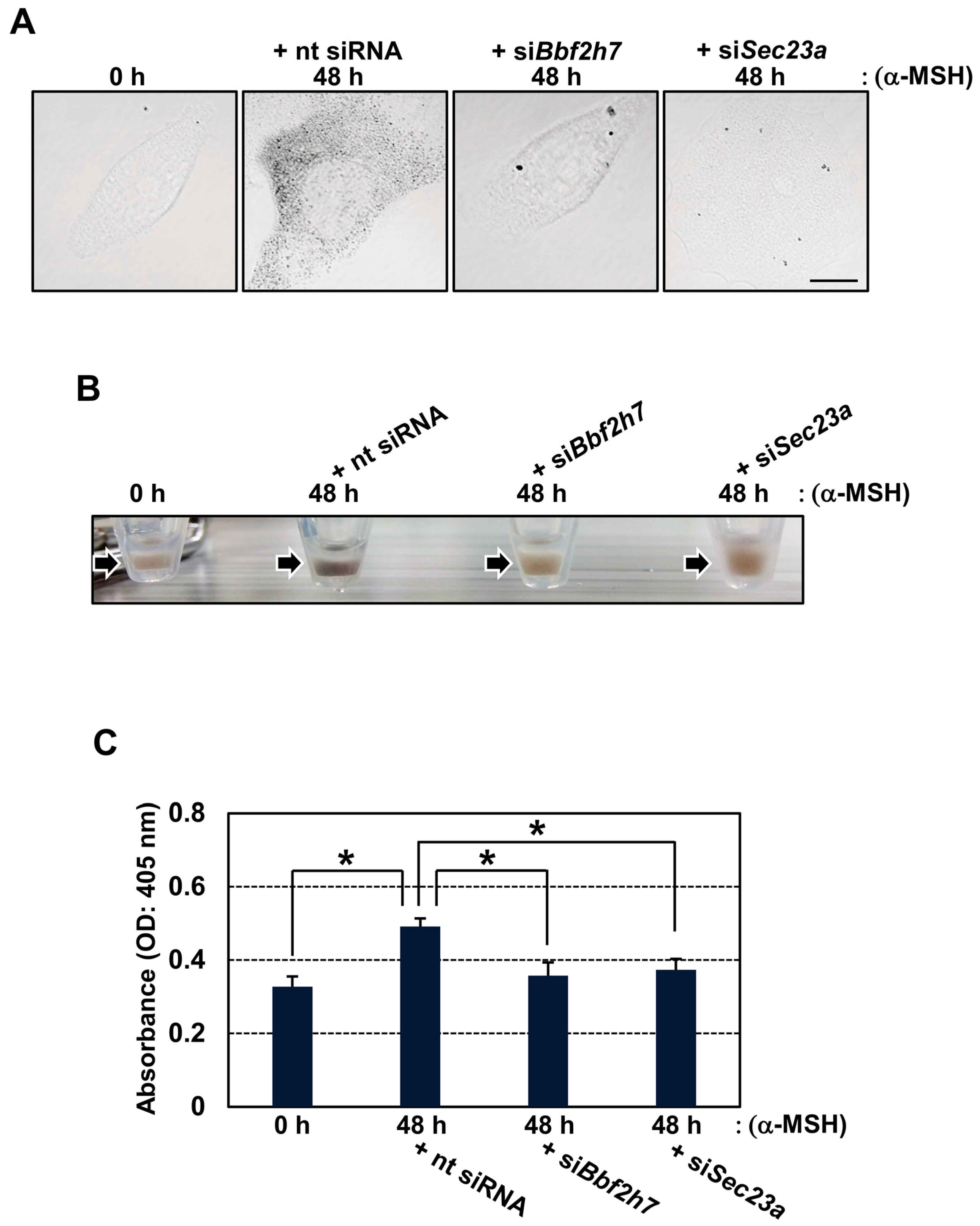
2.3. Inhibition of BBF2H7-Mediated Vesicular Transport Suppresses Melanin Production
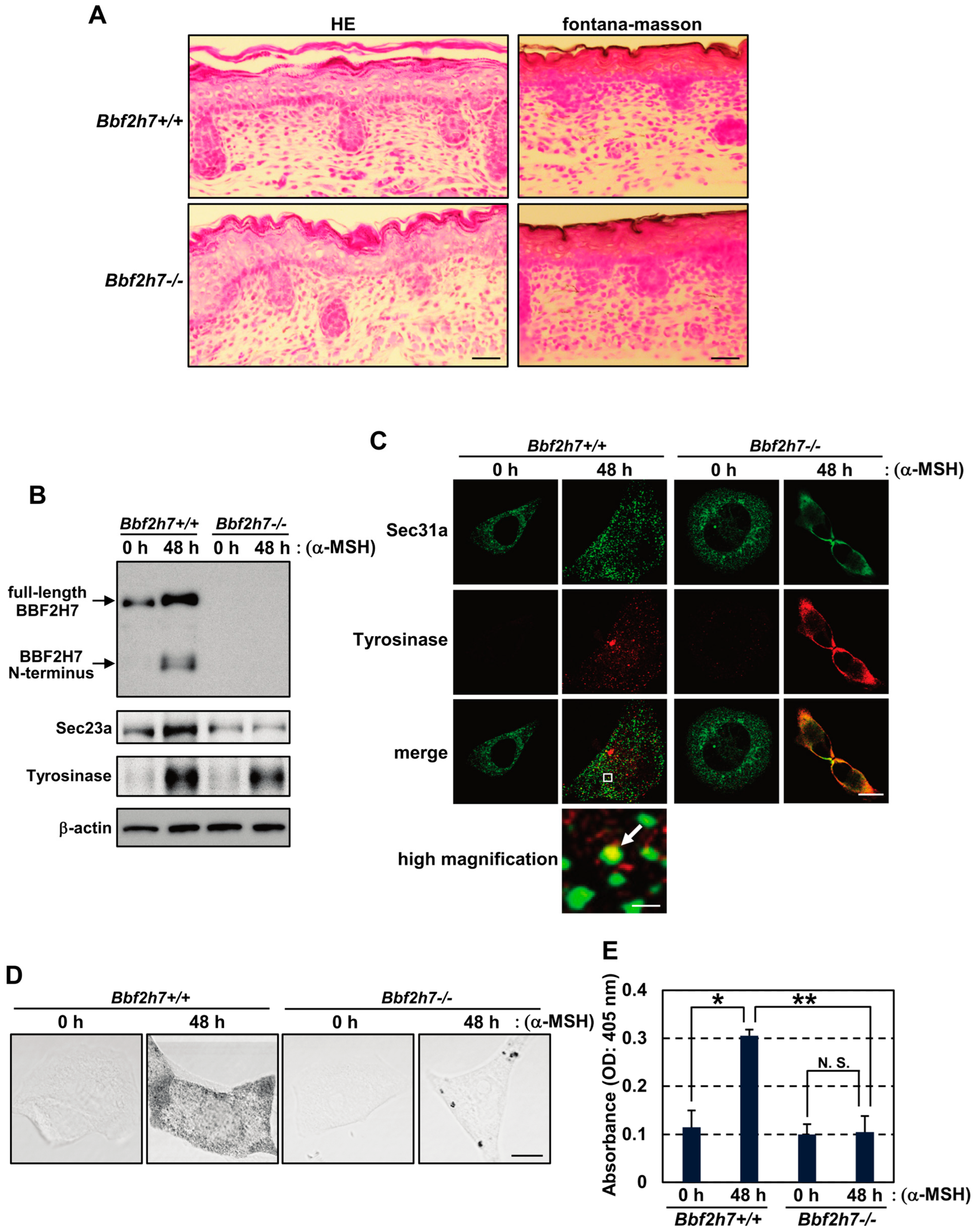
2.4. Restoration of the BBF2H7-Sec23a Pathway Promotes Vesicular Transport of Tyrosinase and Melanin Production
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Cell Culture and Treatments
4.3. Protein Preparation and Western Blotting
4.4. Immunofluorescence Staining
4.5. Chromatin Immunoprecipitation Assay
4.6. Melanin Content Assay
4.7. Histological Analysis
4.8. Data Collection
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elias, P.M. The skin barrier as an innate immune element. Semin. Immunopathol. 2007, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Madison, K.C. Barrier function of the skin: “la raison d’être” of the epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef]
- Elias, P.M. Stratum corneum defensive functions: An integrated view. J. Investig. Dermatol. 2005, 125, 183–200. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An update of the defensive barrier function of skin. Yonsei Med. J. 2006, 47, 293–306. [Google Scholar] [CrossRef]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef]
- Kobayashi, N.; Nakagawa, A.; Muramatsu, T.; Yamashina, Y.; Shirai, T.; Hashimoto, M.W.; Ishigaki, Y.; Ohnishi, T.; Mori, T. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J. Investig. Dermatol. 1998, 110, 806–810. [Google Scholar] [CrossRef]
- Boissy, R.E.; Nordlund, J.J. Molecular basis of congenital hypopigmentary disorders in humans: A review. Pigment Cell Res. 1997, 10, 12–24. [Google Scholar] [CrossRef]
- Frisoli, M.L.; Essien, K.; Harris, J.E. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu. Rev. Immunol. 2020, 38, 621–648. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.Z.; Zhou, A.E.; Hoegler, K.M.; Khachemoune, A. Oculocutaneous albinism: Epidemiology, genetics, skin manifestation, and psychosocial issues. Arch. Dermatol. Res. 2023, 315, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Bolognia, J.; Murray, M.; Pawelek, J. UVB-induced melanogenesis may be mediated through the MSH-receptor system. J. Investig. Dermatol. 1989, 92, 651–656. [Google Scholar] [CrossRef]
- Schauer, E.; Trautinger, F.; Köck, A.; Schwarz, A.; Bhardwaj, R.; Simon, M.; Ansel, J.C.; Schwarz, T.; Luger, T.A. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J. Clin. Investig. 1994, 93, 2258–2262. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Bentley, N.J.; Eisen, T.; Goding, C.R. Melanocyte-specific expression of the human tyrosinase promoter: Activation by the microphthalmia gene product and role of the initiator. Mol. Cell. Biol. 1994, 14, 7996–8006. [Google Scholar] [PubMed]
- Marks, M.S.; Seabra, M.C. The melanosome: Membrane dynamics in black and white. Nat. Rev. Mol. Cell Biol. 2001, 2, 738–748. [Google Scholar] [CrossRef]
- Branza-Nichita, N.; Negroiu, G.; Petrescu, A.J.; Garman, E.F.; Platt, F.M.; Wormald, M.R.; Dwek, R.A.; Petrescu, S.M. Mutations at critical N-glycosylation sites reduce tyrosinase activity by altering folding and quality control. J. Biol. Chem. 2000, 275, 8169–8175. [Google Scholar] [CrossRef]
- Land, E.J.; Riley, P.A. Spontaneous redox reactions of dopaquinone and the balance between the eumelanic and phaeomelanic pathways. Pigment Cell Res. 2000, 13, 273–277. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, A.; Omura, I.; Kamikawa, Y.; Hide, M.; Tanaka, A.; Kaneko, M.; Imaizumi, K.; Saito, A. Unfolded protein response modulates Tyrosinase levels and melanin production during melanogenesis. J. Dermatol. Sci. 2025, 117, 36–44. [Google Scholar] [CrossRef]
- Tirasophon, W.; Welihinda, A.A.; Kaufman, R.J. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998, 12, 1812–1824. [Google Scholar] [CrossRef]
- Yoshida, H.; Okada, T.; Haze, K.; Yanagi, H.; Yura, T.; Negishi, M.; Mori, K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 2000, 20, 6755–6767. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274, Erratum in Nature 1999, 398, 90. [Google Scholar] [CrossRef]
- Ron, D. Translational control in the endoplasmic reticulum stress response. J. Clin. Investig. 2002, 110, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Kaufman, R.J. ER stress and the unfolded protein response. Mutat. Res. 2005, 569, 29–63. [Google Scholar] [CrossRef]
- Saito, A.; Kanemoto, S.; Kawasaki, N.; Asada, R.; Iwamoto, H.; Oki, M.; Miyagi, H.; Izumi, S.; Sanosaka, T.; Nakashima, K.; et al. Unfolded protein response, activated by OASIS family transcription factors, promotes astrocyte differentiation. Nat. Commun. 2012, 3, 967. [Google Scholar] [CrossRef]
- Saito, A.; Hino, S.; Murakami, T.; Kanemoto, S.; Kondo, S.; Saitoh, M.; Nishimura, R.; Yoneda, T.; Furuichi, T.; Ikegawa, S.; et al. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat. Cell Biol. 2009, 11, 1197–1204. [Google Scholar] [CrossRef]
- Murakami, T.; Saito, A.; Hino, S.; Kondo, S.; Kanemoto, S.; Chihara, K.; Sekiya, H.; Tsumagari, K.; Ochiai, K.; Yoshinaga, K.; et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat. Cell Biol. 2009, 11, 1205–1211. [Google Scholar] [CrossRef]
- Asada, R.; Saito, A.; Kawasaki, N.; Kanemoto, S.; Iwamoto, H.; Oki, M.; Miyagi, H.; Izumi, S.; Imaizumi, K. The endoplasmic reticulum stress transducer OASIS is involved in the terminal differentiation of goblet cells in the large intestine. J. Biol. Chem. 2012, 287, 8144–8153. [Google Scholar] [CrossRef]
- Iwakoshi, N.N.; Lee, A.H.; Glimcher, L.H. The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response. Immunol. Rev. 2003, 194, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Saito, A. Physiological functions of endoplasmic reticulum stress transducer OASIS in central nervous system. Anat. Sci. Int. 2014, 89, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Kanemoto, S.; Zhang, Y.; Asada, R.; Hino, K.; Imaizumi, K. Chondrocyte proliferation regulated by secreted luminal domain of ER stress transducer BBF2H7/CREB3L2. Mol. Cell 2014, 53, 127–139. [Google Scholar] [CrossRef]
- Ito, T.; Saito, A.; Kamikawa, Y.; Nakazawa, N.; Imaizumi, K. AIbZIP/CREB3L4 Promotes Cell Proliferation via the SKP2-p27 Axis in Luminal Androgen Receptor Subtype Triple-Negative Breast Cancer. Mol. Cancer Res. 2024, 22, 373–385. [Google Scholar] [CrossRef]
- Lee, A.H.; Scapa, E.F.; Cohen, D.E.; Glimcher, L.H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 2008, 320, 1492–1496. [Google Scholar] [CrossRef]
- Yu, Y.; Pierciey, F.J., Jr.; Maguire, T.G.; Alwine, J.C. PKR-like endoplasmic reticulum kinase is necessary for lipogenic activation during HCMV infection. PLoS Pathog. 2013, 9, e1003266. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takahara, K.; Oyadomari, S.; Okada, T.; Sato, T.; Harada, A.; Mori, K. Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol. Biol. Cell 2010, 21, 2975–2986. [Google Scholar] [CrossRef]
- Sue, N.; Thai, L.M.; Saito, A.; Boyer, C.K.; Fordham, A.M.; Yan, C.; Davenport, A.; Tao, J.; Bensellam, M.; Cantley, J.; et al. Independent activation of CREB3L2 by glucose fills a regulatory gap in mouse β-cells by co-ordinating insulin biosynthesis with secretory granule formation. Mol. Metab. 2024, 79, 101845. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Kamikawa, Y.; Ito, T.; Matsuhisa, K.; Kaneko, M.; Okamoto, T.; Yoshimaru, T.; Matsushita, Y.; Katagiri, T.; Imaizumi, K. p53-independent tumor suppression by cell-cycle arrest via CREB/ATF transcription factor OASIS. Cell Rep. 2023, 42, 112479. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Omura, I.; Imaizumi, K. CREB3L1/OASIS: Cell cycle regulator and tumor suppressor. FEBS J. 2024, 291, 4853–4866. [Google Scholar] [CrossRef]
- González-Quiroz, M.; Blondel, A.; Sagredo, A.; Hetz, C.; Chevet, E.; Pedeux, R. When Endoplasmic Reticulum Proteostasis Meets the DNA Damage Response. Trends Cell Biol. 2020, 30, 881–891. [Google Scholar] [CrossRef]
- Kondo, S.; Saito, A.; Hino, S.; Murakami, T.; Ogata, M.; Kanemoto, S.; Nara, S.; Yamashita, A.; Yoshinaga, K.; Hara, H.; et al. BBF2H7, a novel transmembrane bZIP transcription factor, is a new type of endoplasmic reticulum stress transducer. Mol. Cell. Biol. 2007, 27, 1716–1729. [Google Scholar] [CrossRef]
- Izumi, S.; Saito, A.; Kanemoto, S.; Kawasaki, N.; Asada, R.; Iwamoto, H.; Oki, M.; Miyagi, H.; Ochi, M.; Imaizumi, K. The endoplasmic reticulum stress transducer BBF2H7 suppresses apoptosis by activating the ATF5-MCL1 pathway in growth plate cartilage. J. Biol. Chem. 2012, 287, 36190–36200. [Google Scholar] [CrossRef] [PubMed]
- Mica, Y.; Lee, G.; Chambers, S.M.; Tomishima, M.J.; Studer, L. Modeling neural crest induction, melanocyte specification, and disease-related pigmentation defects in hESCs and patient-specific iPSCs. Cell Rep. 2013, 3, 1140–1152. [Google Scholar] [CrossRef]
- Singh, A.; Gotherwal, V.; Junni, P.; Vijayan, V.; Tiwari, M.; Ganju, P.; Kumar, A.; Sharma, P.; Fatima, T.; Gupta, A.; et al. Mapping architectural and transcriptional alterations in non-lesional and lesional epidermis in vitiligo. Sci. Rep. 2017, 7, 9860. [Google Scholar] [CrossRef]
- Fromme, J.C.; Orci, L.; Schekman, R. Coordination of COPII vesicle trafficking by Sec23. Trends Cell Biol. 2008, 18, 330–336. [Google Scholar] [CrossRef]
- Paccaud, J.P.; Reith, W.; Carpentier, J.L.; Ravazzola, M.; Amherdt, M.; Schekman, R.; Orci, L. Cloning and functional characterization of mammalian homologues of the COPII component Sec23. Mol. Biol. Cell 1996, 7, 1535–1546. [Google Scholar] [CrossRef]
- Hirobe, T. Histochemical survey of the distribution of the epidermal melanoblasts and melanocytes in the mouse during fetal and postnatal periods. Anat. Rec. 1984, 208, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Yajima, I.; Kumasaka, M.Y.; Iida, M.; Oshino, R.; Tanihata, H.; Al Hossain, A.; Ohgami, N.; Kato, M. Arsenic-mediated hyperpigmentation in skin via NF-kappa B/endothelin-1 signaling in an originally developed hairless mouse model. Arch. Toxicol. 2017, 91, 3507–3516. [Google Scholar] [CrossRef]
- Yoshida, H.; Kunisada, T.; Grimm, T.; Nishimura, E.K.; Nishioka, E.; Nishikawa, S.I. Review: Melanocyte migration and survival controlled by SCF/c-kit expression. J. Investig. Dermatol. Symp. Proc. 2001, 6, 1–5. [Google Scholar] [CrossRef]
- Bommiasamy, H.; Back, S.H.; Fagone, P.; Lee, K.; Meshinchi, S.; Vink, E.; Sriburi, R.; Frank, M.; Jackowski, S.; Kaufman, R.J.; et al. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J. Cell Sci. 2009, 122, 1626–1636. [Google Scholar] [CrossRef]
- Calfon, M.; Zeng, H.; Urano, F.; Till, J.H.; Hubbard, S.R.; Harding, H.P.; Clark, S.G.; Ron, D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002, 415, 92–96, Correction in Nature 2002, 420, 202. [Google Scholar] [CrossRef] [PubMed]
- Veit, G.; Avramescu, R.G.; Chiang, A.N.; Houck, S.A.; Cai, Z.; Peters, K.W.; Hong, J.S.; Pollard, H.B.; Guggino, W.B.; Balch, W.E.; et al. From CFTR biology toward combinatorial pharmacotherapy: Expanded classification of cystic fibrosis mutations. Mol. Biol. Cell 2016, 27, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Osaki, Y.; Matsuhisa, K.; Che, W.; Kaneko, M.; Asada, R.; Masaki, T.; Imaizumi, K.; Saito, A. Calnexin promotes the folding of mutant iduronate 2-sulfatase related to mucopolysaccharidosis type II. Biochem. Biophys. Res. Commun. 2019, 514, 217–223. [Google Scholar] [CrossRef]
- Osaki, Y.; Saito, A.; Kanemoto, S.; Kaneko, M.; Matsuhisa, K.; Asada, R.; Masaki, T.; Orii, K.; Fukao, T.; Tomatsu, S.; et al. Shutdown of ER-associated degradation pathway rescues functions of mutant iduronate 2-sulfatase linked to mucopolysaccharidosis type II. Cell Death Dis. 2018, 9, 808. [Google Scholar] [CrossRef]
- Guan, M.; Fousek, K.; Chow, W.A. Nelfinavir inhibits regulated intramembrane proteolysis of sterol regulatory element binding protein-1 and activating transcription factor 6 in castration-resistant prostate cancer. FEBS J. 2012, 279, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.A.; Brown, M.S.; Goldstein, J.L.; Sakai, J. Cleavage site for sterol-regulated protease localized to a leu-Ser bond in the lumenal loop of sterol regulatory element-binding protein-2. J. Biol. Chem. 1997, 272, 12778–12785. [Google Scholar] [CrossRef]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Davé, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Rawson, R.B.; Zelenski, N.G.; Nijhawan, D.; Ye, J.; Sakai, J.; Hasan, M.T.; Chang, T.Y.; Brown, M.S.; Goldstein, J.L. Complementation cloning of S2P, a gene encoding a putative metalloprotease required for intramembrane cleavage of SREBPs. Mol. Cell 1997, 1, 47–57. [Google Scholar] [CrossRef]
- Indra, A.K.; Warot, X.; Brocard, J.; Bornert, J.M.; Xiao, J.H.; Chambon, P.; Metzger, D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: Comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999, 27, 4324–4327. [Google Scholar] [CrossRef]
- Hirobe, T. Basic fibroblast growth factor stimulates the sustained proliferation of mouse epidermal melanoblasts in a serum-free medium in the presence of dibutyryl cyclic AMP and keratinocytes. Development 1992, 114, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, T.; Ishikawa, A. l-tyrosine induces melanocyte differentiation in novel pink-eyed dilution castaneus mouse mutant showing age-related pigmentation. J. Dermatol. Sci. 2015, 80, 203–211. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, M.; Wildenhain, J.; Rappsilber, J.; Tyers, M. BoxPlotR: A web tool for generation of box plots. Nat. Methods 2014, 11, 121–122. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Phan, G.H.; Fujise, K.; Imaizumi, K.; Saito, A. Vesicular Transport Mediated by Endoplasmic Reticulum Stress Sensor BBF2H7 Orchestrates Melanin Production During Melanogenesis. Int. J. Mol. Sci. 2026, 27, 501. https://doi.org/10.3390/ijms27010501
Phan GH, Fujise K, Imaizumi K, Saito A. Vesicular Transport Mediated by Endoplasmic Reticulum Stress Sensor BBF2H7 Orchestrates Melanin Production During Melanogenesis. International Journal of Molecular Sciences. 2026; 27(1):501. https://doi.org/10.3390/ijms27010501
Chicago/Turabian StylePhan, Giang Huy, Kenshiro Fujise, Kazunori Imaizumi, and Atsushi Saito. 2026. "Vesicular Transport Mediated by Endoplasmic Reticulum Stress Sensor BBF2H7 Orchestrates Melanin Production During Melanogenesis" International Journal of Molecular Sciences 27, no. 1: 501. https://doi.org/10.3390/ijms27010501
APA StylePhan, G. H., Fujise, K., Imaizumi, K., & Saito, A. (2026). Vesicular Transport Mediated by Endoplasmic Reticulum Stress Sensor BBF2H7 Orchestrates Melanin Production During Melanogenesis. International Journal of Molecular Sciences, 27(1), 501. https://doi.org/10.3390/ijms27010501







