Mitophagy–NLRP3 Inflammasome Crosstalk in Parkinson’s Disease: Pathogenic Mechanisms and Emerging Therapeutic Strategies
Abstract
1. Introduction
2. Inflammasomes Expression, Activation and Regulation
2.1. Inflammatory Mechanisms in the Brain
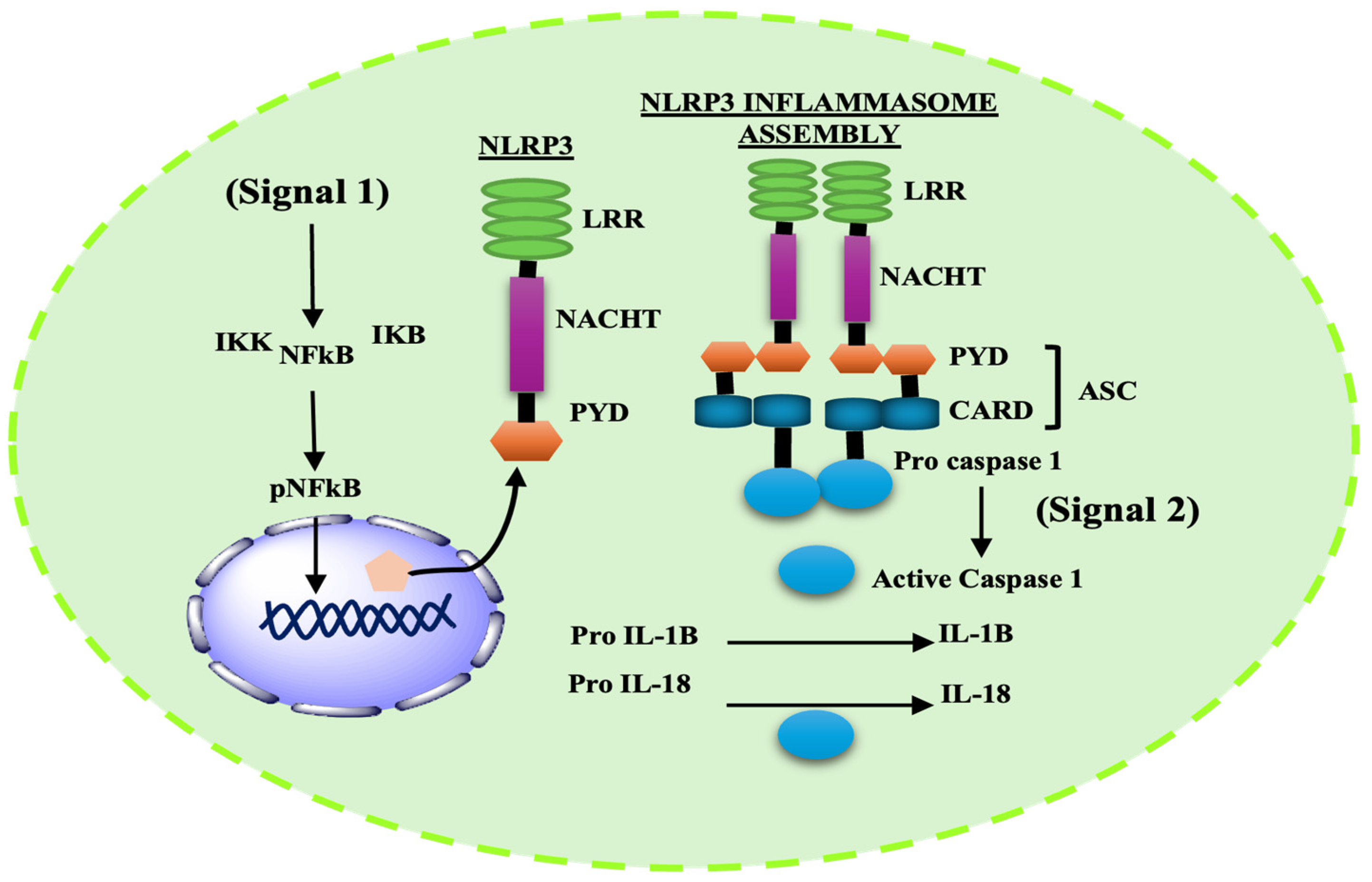
2.2. Inflammasome Complex: Structure and Function
2.3. Structure and Activation Mechanism of NLRP3 Inflammasome
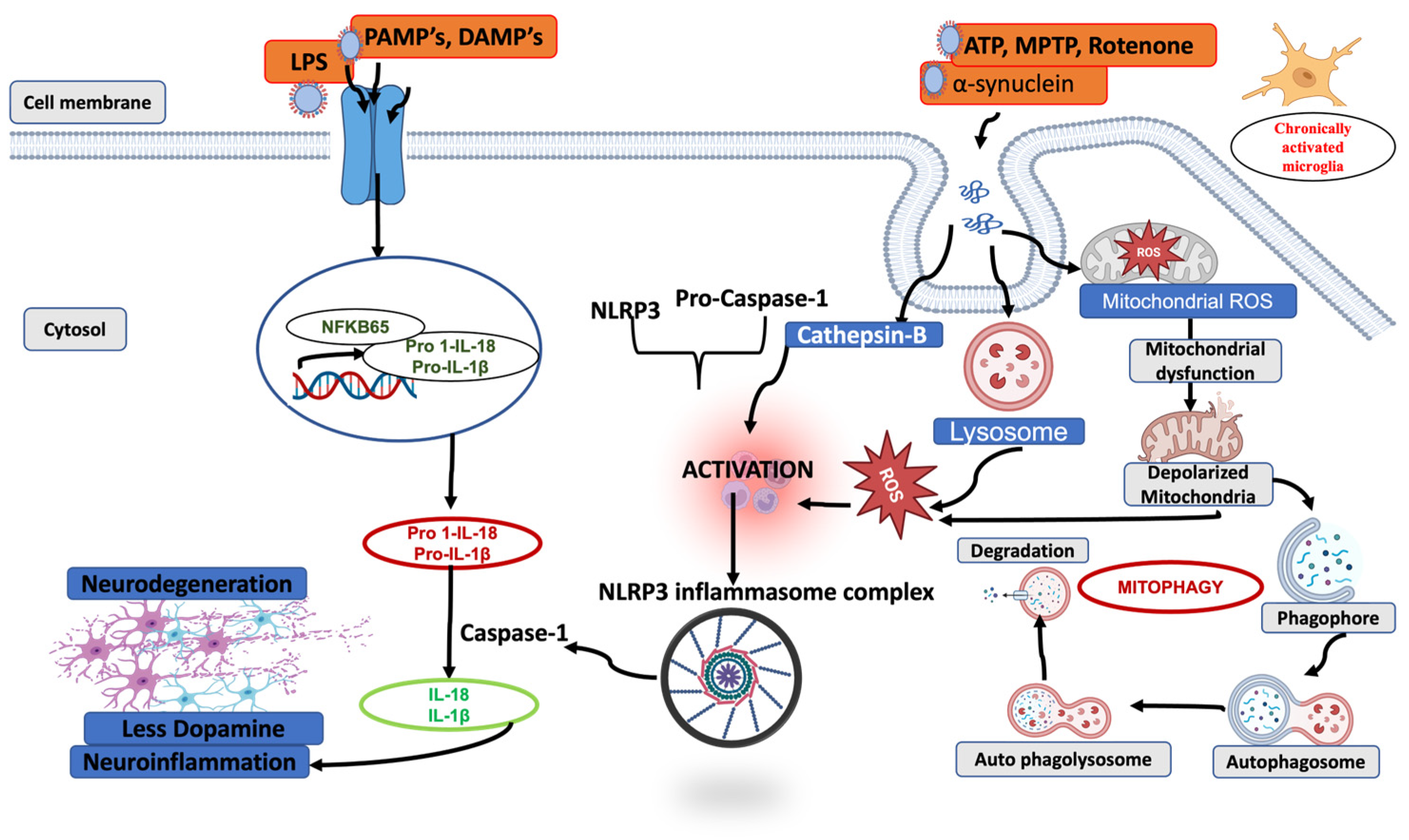
3. NLRP3 Inflammasomes in Parkinson’s Disease
4. General View of Autophagy and Its Activation
5. Mitophagy-Mediated Control of Mitochondrial Homeostasis and NLRP3 Inflammasomes Regulation
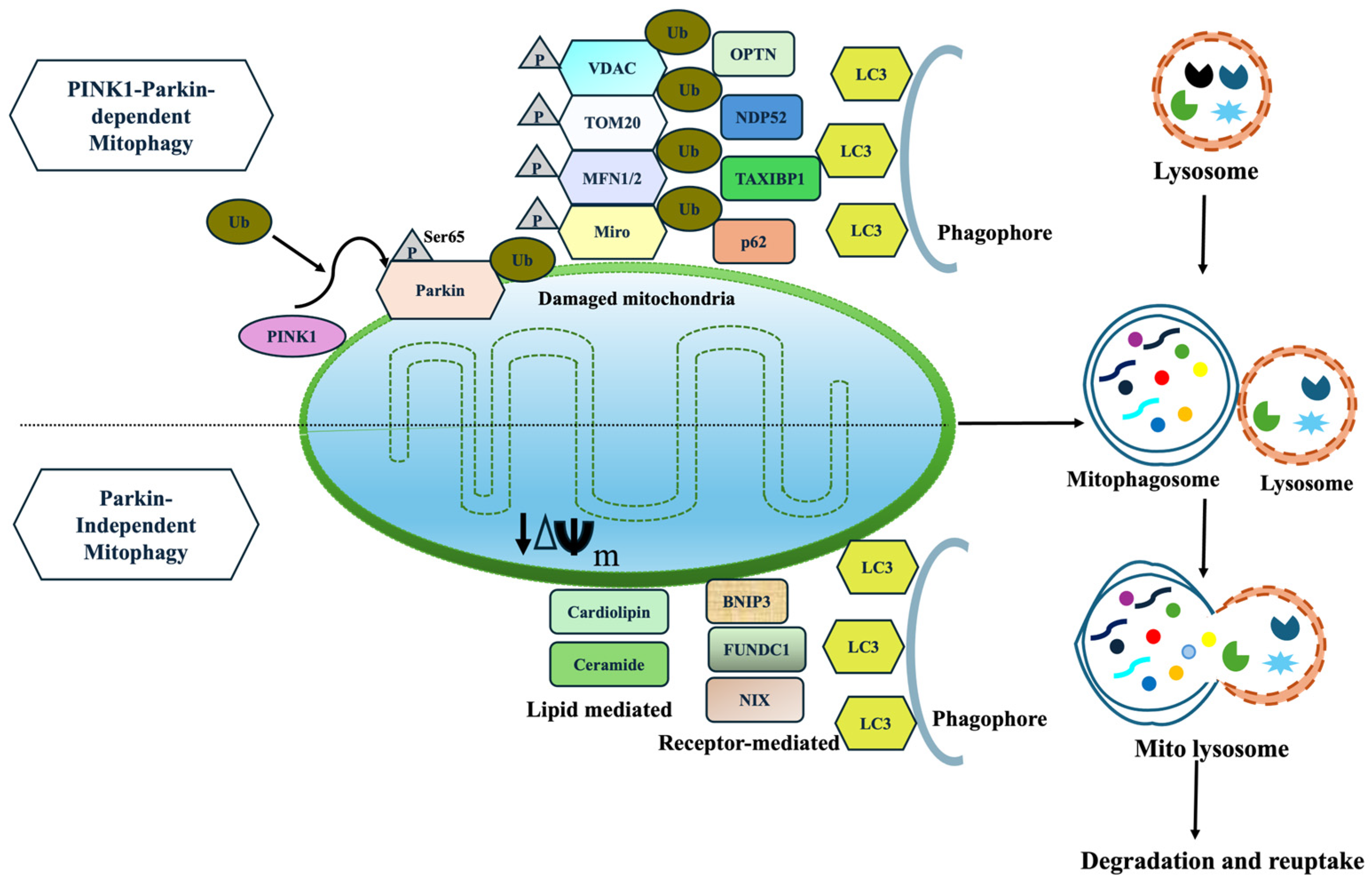
5.1. Mitophagy
5.1.1. PINK1 and Parkin-Dependent Mitophagy
5.1.2. Parkin-Independent and Receptor-Mediated Mitophagy
5.2. Direct Degradation of Inflammasomes Through Autophagy
6. Drug Targets
6.1. Autophagy/Mitophagy Enhancers
6.2. NLRP3 Inhibitors
6.3. Dual Modulation
6.4. Drugs in Clinical Trials
7. Limitations of Current Therapeutic Strategies
8. Conclusions
9. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| SNpc | Substantia Nigra Par Compacta |
| CNS | Central Nervous System |
| HLA-DR | Human Leukocyte Antigen-D-Related |
| DAMPS | Damage-Associated Molecular Patterns |
| ROS | Reactive Oxygen Species |
| IL-1β | Interleukin-1 beta) |
| Il-18 | Interleukin-18 |
| NLRP3 | NOD-, LRR-, and Pyrin Domain-Containing Protein 3 |
| PAMPs | Pathogen-Associated Molecular Patterns |
| MPTP | 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine |
| PYHIN/HIN | Hematopoietic Interferon-Inducible Nuclear |
| AIM2 | Absent in Melanoma 2 |
| IFI16 | Interferon-Gamma Inducible Protein 16 |
| ATP | Adenosine Triphosphate |
| NACHT | Nucleotide-Binding |
| LRR | Leucine Rich Repeats |
| CARD | Caspase Recruitment Domain |
| TLR | Toll-like Receptor |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| TNF | Tumor Necrosis Factor |
| CSF | Cerebrospinal Fluid |
| AD | Alzheimer’s Disease |
| SNP | Single-Nucleotide Polymorphisms |
| PARP | Poly (ADP-ribose) Polymerase 1 |
| iPSC | Induced Pluripotent Stem Cell |
| mtDNA | Mitochondrial DNA |
| CMA | Chaperone-Mediated Autophagy |
| LAMP2A | Lysosomal-Associated Membrane Protein 2 |
| ULK1 | Unc-51-Like Autophagy Activating Kinase 1 |
| mTORC1 | Mechanistic Target of Rapamycin Complex 1 |
| LC3I | Microtubule-associated protein 1 light chain 3 |
| ATG | Autophagy-Related |
| SQSTM1 | Sequestosome 1 |
| PINK1 | PTEN-Induced Putative Kinase 1 |
| OMM | Outer Mitochondrial Membrane |
| OPTN | Optineurin |
| FUNDC1 | FUN14 Domain Containing 1 |
| OPA1 | Optic Atrophy 1 |
| BNIP3 | Bcl-2/Adenovirus E1B 19-kDa-Interacting Protein 3 |
| LPS | Lipopolysaccharide |
| AMPK | AMP-Activated Protein Kinase |
| fMRI | Functional Magnetic Resonance Imaging |
| PET | Positron Emission Tomography |
| TSPO | Translocator Protein |
References
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The epidemiology of Parkinson’s disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef]
- Xu, T.; Dong, W.; Liu, J.; Yin, P.; Wang, Z.; Zhang, L.; Zhou, M. Disease burden of Parkinson’s disease in China and its provinces from 1990 to 2021: Findings from the global burden of disease study 2021. Lancet Reg. Health–West. Pac. 2024, 46, 101078. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.A.; Romero-Ramos, M. Microglia response during Parkinson’s Disease: Alpha-synuclein intervention. Front. Cell. Neurosci. 2018, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Guo, D.; Wei, J. The pathogenesis of Parkinson’s disease and crosstalk with other diseases. Biocell 2024, 48, 1155. [Google Scholar] [CrossRef]
- Sabbagh, M.; Small, G.W.; Isaacson, S.H.; Torres-Yaghi, Y.; Pagan, F.; Pahwa, R. Unmet needs in the diagnosis and treatment of Parkinson’s disease psychosis and dementia-related psychosis. Int. J. Psychiatry Clin. Pract. 2023, 27, 69–81. [Google Scholar] [CrossRef]
- Leak, R.K.; Clark, R.N.; Abbas, M.; Xu, F.; Brodsky, J.L.; Chen, J.; Hu, X.; Luk, K.C. Current insights and assumptions on α-synuclein in Lewy body disease. Acta Neuropathol. 2024, 148, 18. [Google Scholar] [CrossRef]
- Runwal, G.; Edwards, R.H. The membrane interactions of synuclein: Physiology and pathology. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 465–485. [Google Scholar] [CrossRef]
- Calabresi, P.; Di Lazzaro, G.; Marino, G.; Campanelli, F.; Ghiglieri, V. Advances in understanding the function of alpha-synuclein: Implications for Parkinson’s disease. Brain 2023, 146, 3587–3597. [Google Scholar] [CrossRef]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J. Aging and Parkinson’s disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef]
- Cucinotta, L.; Mannino, D.; Filippone, A.; Romano, A.; Esposito, E.; Paterniti, I. The role of autophagy in Parkinson’s disease: A gender difference overview. Front. Pharmacol. 2024, 15, 1408152. [Google Scholar] [CrossRef]
- Sarkar, S.; Malovic, E.; Harishchandra, D.S.; Ghaisas, S.; Panicker, N.; Charli, A.; Palanisamy, B.N.; Rokad, D.; Jin, H.; Anantharam, V. Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson’s disease. NPJ Park. Dis. 2017, 3, 30. [Google Scholar] [CrossRef]
- Zhu, B.; Yin, D.; Zhao, H.; Zhang, L. The immunology of Parkinson’s disease. Semin. Immunopathol. 2022, 44, 659–672. [Google Scholar] [CrossRef]
- Roodveldt, C.; Bernardino, L.; Oztop-Cakmak, O.; Dragic, M.; Fladmark, K.E.; Ertan, S.; Aktas, B.; Pita, C.; Ciglar, L.; Garraux, G. The immune system in Parkinson’s disease: What we know so far. Brain 2024, 147, 3306–3324. [Google Scholar] [CrossRef]
- Neubrand, V.E.; Sepúlveda, M.R. New insights into the role of the endoplasmic reticulum in microglia. Neural Regen. Res. 2024, 19, 1397–1398. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Polis, B.; Jamwal, S.; Sanganahalli, B.G.; Kaswan, Z.M.; Islam, R.; Kim, D.; Bowers, C.; Giuliano, L.; Biederer, T. Transient impairment in microglial function causes sex-specific deficits in synaptic maturity and hippocampal function in mice exposed to early adversity. Brain Behav. Immun. 2024, 122, 95–109. [Google Scholar] [CrossRef]
- Lind-Holm Mogensen, F.; Seibler, P.; Grünewald, A.; Michelucci, A. Microglial dynamics and neuroinflammation in prodromal and early Parkinson’s disease. J. Neuroinflamm. 2025, 22, 136. [Google Scholar] [CrossRef]
- McGeer, P.; Itagaki, S.; Boyes, B.; McGeer, E. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, G.; Hutchinson, H.; Gonzalez, M.; Dagra, A. Central and Peripheral Immunity Responses in Parkinson’s Disease: An Overview and Update. Neuroglia 2025, 6, 17. [Google Scholar] [CrossRef]
- Xu, X.; Yin, D.; Ren, H.; Gao, W.; Li, F.; Sun, D.; Wu, Y.; Zhou, S.; Lyu, L.; Yang, M. Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury. Neurobiol. Dis. 2018, 117, 15–27. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Polis, B.; Kaffman, A. Microglia: The Drunken Gardeners of Early Adversity. Biomolecules 2024, 14, 964. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Tian, M.; Deng, S.; Li, J.; Yang, M.; Gao, J.; Pei, X.; Wang, Y.; Tan, J.; Zhao, F. The key drivers of brain injury by systemic inflammatory responses after sepsis: Microglia and neuroinflammation. Mol. Neurobiol. 2023, 60, 1369–1390. [Google Scholar] [CrossRef]
- Dayananda, K.K.; Ahmed, S.; Wang, D.; Polis, B.; Islam, R.; Kaffman, A. Early life stress impairs synaptic pruning in the developing hippocampus. Brain Behav. Immun. 2023, 107, 16–31. [Google Scholar] [CrossRef]
- Liu, T.-W.; Chen, C.-M.; Chang, K.-H. Biomarker of neuroinflammation in Parkinson’s disease. Int. J. Mol. Sci. 2022, 23, 4148. [Google Scholar] [CrossRef]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kwatra, M.; Gawali, B.; Panda, S.R.; Naidu, V. Potential role of TrkB agonist in neuronal survival by promoting CREB/BDNF and PI3K/Akt signaling in vitro and in vivo model of 3-nitropropionic acid (3-NP)-induced neuronal death. Apoptosis 2021, 26, 52–70. [Google Scholar] [CrossRef]
- Nakaso, K. Roles of Microglia in Neurodegenerative Diseases. Yonago Acta Medica 2024, 67, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, W.; Sun, Y.; Wu, M. New insight on microglia activation in neurodegenerative diseases and therapeutics. Front. Neurosci. 2023, 17, 1308345. [Google Scholar] [CrossRef]
- Nechushtai, L.; Frenkel, D.; Pinkas-Kramarski, R. Autophagy in Parkinson’s disease. Biomolecules 2023, 13, 1435. [Google Scholar] [CrossRef]
- Pradas, E.; Martinez-Vicente, M. The consequences of GBA deficiency in the autophagy–lysosome system in Parkinson’s disease associated with GBA. Cells 2023, 12, 191. [Google Scholar] [CrossRef]
- Hussain, M.S.; Moglad, E.; Afzal, M.; Sharma, S.; Gupta, G.; Sivaprasad, G.; Deorari, M.; Almalki, W.H.; Kazmi, I.; Alzarea, S.I. Autophagy-associated non-coding RNAs: Unraveling their impact on Parkinson’s disease pathogenesis. CNS Neurosci. Ther. 2024, 30, e14763. [Google Scholar] [CrossRef] [PubMed]
- Hull, A.; Atilano, M.L.; Gergi, L.; Kinghorn, K.J. Lysosomal storage, impaired autophagy and innate immunity in Gaucher and Parkinson’s diseases: Insights for drug discovery. Philos. Trans. R. Soc. B 2024, 379, 20220381. [Google Scholar] [CrossRef] [PubMed]
- Usenko, T. Autophagy impairment in Parkinson’s disease: Approaches to therapy. Mol. Biol. 2025, 59, 51–68. [Google Scholar] [CrossRef]
- Wang, B.; Abraham, N.; Gao, G.; Yang, Q. Dysregulation of autophagy and mitochondrial function in Parkinson’s disease. Transl. Neurodegener. 2016, 5, 19. [Google Scholar] [CrossRef]
- Bagwell, E.; Larsen, J. A review of MPTP-induced parkinsonism in adult zebrafish to explore pharmacological interventions for human Parkinson’s disease. Front. Neurosci. 2024, 18, 1451845. [Google Scholar] [CrossRef]
- Antico, O.; Thompson, P.W.; Hertz, N.T.; Muqit, M.M.; Parton, L.E. Targeting mitophagy in neurodegenerative diseases. Nat. Rev. Drug Discov. 2025, 24, 276–299. [Google Scholar] [CrossRef]
- Liu, M.; Liu, S.; Lin, Z.; Chen, X.; Jiao, Q.; Du, X.; Jiang, H. Targeting the Interplay Between Autophagy and the Nrf2 Pathway in Parkinson’s Disease with Potential Therapeutic Implications. Biomolecules 2025, 15, 149. [Google Scholar] [CrossRef]
- García-Domínguez, M. Neuroinflammation: Mechanisms, Dual Roles, and Therapeutic Strategies in Neurological Disorders. Curr. Issues Mol. Biol. 2025, 47, 417. [Google Scholar] [CrossRef] [PubMed]
- Verma, O.; Faizan, M.; Goswami, S.; Singh, M.P. An update on the involvement of inflammatory mediators in Parkinson’s disease pathogenesis. Arch. Toxicol. 2025, 99, 3527–3552. [Google Scholar] [CrossRef]
- Jewell, S.; Herath, A.M.; Gordon, R. Inflammasome Activation in Parkinson’s Disease. J. Park. Dis. 2022, 12, S113–S128. [Google Scholar] [CrossRef]
- Nguyen, L.T.N.; Nguyen, H.D.; Kim, Y.J.; Nguyen, T.T.; Lai, T.T.; Lee, Y.K.; Ma, H.-I.; Kim, Y.E. Role of NLRP3 inflammasome in Parkinson’s disease and therapeutic considerations. J. Park. Dis. 2022, 12, 2117–2133. [Google Scholar] [CrossRef]
- Han, Q.-Q.; Le, W. NLRP3 inflammasome-mediated neuroinflammation and related mitochondrial impairment in Parkinson’s disease. Neurosci. Bull. 2023, 39, 832–844. [Google Scholar] [CrossRef]
- Han, Y.-H.; Liu, X.-D.; Jin, M.-H.; Sun, H.-N.; Kwon, T. Role of NLRP3 inflammasome-mediated neuronal pyroptosis and neuroinflammation in neurodegenerative diseases. Inflamm. Res. 2023, 72, 1839–1859. [Google Scholar] [CrossRef] [PubMed]
- Saad, H.M.; Atef, E.; Elsayed, A.E. New Insights on the Potential Role of Pyroptosis in Parkinson’s Neuropathology and Therapeutic Targeting of NLRP3 Inflammasome with Recent Advances in Nanoparticle-Based miRNA Therapeutics. Mol. Neurobiol. 2025, 62, 9365–9384. [Google Scholar] [CrossRef]
- García-Revilla, J.; Herrera, A.J.; De Pablos, R.M.; Venero, J.L. Inflammatory animal models of Parkinson’s disease. J. Park. Dis. 2022, 12, S165–S182. [Google Scholar] [CrossRef]
- Jain, R.; Vora, L.; Nathiya, D.; Khatri, D.K. Nrf2–Keap1 Pathway and NLRP3 Inflammasome in Parkinson’s Disease: Mechanistic Crosstalk and Therapeutic Implications. Mol. Neurobiol. 2026, 63, 91. [Google Scholar] [CrossRef]
- Singh, J.; Habean, M.L.; Panicker, N. Inflammasome assembly in neurodegenerative diseases. Trends Neurosci. 2023, 46, 814–831. [Google Scholar] [CrossRef] [PubMed]
- Cabrera Ranaldi, E.d.R.; Nuytemans, K.; Martinez, A.; Luca, C.C.; Keane, R.W.; de Rivero Vaccari, J.P. Proof-of-principle study of inflammasome signaling proteins as diagnostic biomarkers of the inflammatory response in Parkinson’s disease. Pharmaceuticals 2023, 16, 883. [Google Scholar] [CrossRef] [PubMed]
- Piancone, F.; La Rosa, F.; Marventano, I.; Saresella, M.; Clerici, M. The Role of the Inflammasome in Neurodegenerative Diseases. Molecules 2021, 26, 953. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, L.; Luo, H.; Li, X.; Ruan, Y.; Fan, R.; Si, Z.; Chen, Y.; Li, L.; Liu, Y. The Significance of NLRP Inflammasome in Neuropsychiatric Disorders. Brain Sci. 2022, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, Z.; Song, W.; Zhang, Y. Targeting NLRP3 inflammasome for neurodegenerative disorders. Mol. Psychiatry 2023, 28, 4512–4527, Correction in Mol. Psychiatry 2024, 28, 4933. [Google Scholar] [CrossRef]
- de Araújo, F.M.; Cuenca-Bermejo, L.; Fernández-Villalba, E.; Costa, S.L.; Silva, V.D.A.; Herrero, M.T. Role of Microgliosis and NLRP3 Inflammasome in Parkinson’s Disease Pathogenesis and Therapy. Cell. Mol. Neurobiol. 2022, 42, 1283–1300. [Google Scholar] [CrossRef]
- Xiao, B.; Kuruvilla, J.; Tan, E.-K. Mitophagy and reactive oxygen species interplay in Parkinson’s disease. NPJ Park. Dis. 2022, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, Z.; Fan, B.; Chen, Y.; Zhou, L.; Jiang, B.; Long, H.; Zhong, W.; Li, X.; Li, Y. A selective NLRP3 inflammasome inhibitor attenuates behavioral deficits and neuroinflammation in a mouse model of Parkinson’s disease. J. Neuroimmunol. 2021, 354, 577543. [Google Scholar] [CrossRef]
- Marinescu, A.M.; Machado, V.; Pagano, G.; Muelhardt, N.M.; Kustermann, T.; Mracsko, E.Z.; Brockmann, K.; Shariati, N.; Anzures-Cabrera, J.; Zinnhardt, B. Cerebrospinal Fluid Biomarkers of NLRP3 Pathway, Immune Dysregulation, and Neurodegeneration in Parkinson’s Disease: A Meta-Analysis. Mov. Disord. 2025. Available online: https://movementdisorders.onlinelibrary.wiley.com/doi/10.1002/mds.70103 (accessed on 18 November 2025). [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef]
- Yao, J.; Sterling, K.; Wang, Z.; Zhang, Y.; Song, W. The role of inflammasomes in human diseases and their potential as therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 10. [Google Scholar] [CrossRef]
- Xu, J.; Núñez, G. The NLRP3 inflammasome: Activation and regulation. Trends Biochem. Sci. 2023, 48, 331–344. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z.; et al. The NLRP3 inflammasome: Contributions to inflammation-related diseases. Cell. Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, Z.; Li, Y.; Chen, J.; Huang, N.; Luo, Y. Role of NLRP3 in Parkinson’s disease: Specific activation especially in dopaminergic neurons. Heliyon 2024, 10, e28838. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Yuan, X.-L.; Jiang, J.-M.; Zhang, P.; Tan, K. Targeting the NLRP3 inflammasome in Parkinson’s disease: From molecular mechanism to therapeutic strategy. Exp. Neurol. 2025, 386, 115167. [Google Scholar] [CrossRef]
- Xu, W.; Huang, Y.; Zhou, R. NLRP3 inflammasome in neuroinflammation and central nervous system diseases. Cell. Mol. Immunol. 2025, 22, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Song, N.; Liu, Z.-S.; Xue, W.; Bai, Z.-F.; Wang, Q.-Y.; Dai, J.; Liu, X.; Huang, Y.-J.; Cai, H.; Zhan, X.-Y. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol. Cell 2017, 68, 185–197.e6. [Google Scholar] [CrossRef] [PubMed]
- Juliana, C.; Fernandes-Alnemri, T.; Kang, S.; Farias, A.; Qin, F.; Alnemri, E.S. Non-transcriptional Priming and Deubiquitination Regulate NLRP3 Inflammasome Activation. J. Biol. Chem. 2012, 287, 36617–36622. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Kim, J.K.; Shin, H.J.; Park, E.-J.; Kim, I.S.; Jo, E.-K. Updated insights into the molecular networks for NLRP3 inflammasome activation. Cell. Mol. Immunol. 2025, 22, 563–596. [Google Scholar] [CrossRef]
- Ye, T.; Tao, W.-Y.; Chen, X.-Y.; Jiang, C.; Di, B.; Xu, L.-L. Mechanisms of NLRP3 inflammasome activation and the development of peptide inhibitors. Cytokine Growth Factor Rev. 2023, 74, 1–13. [Google Scholar] [CrossRef]
- Vidović, M.; Rikalovic, M.G. Alpha-Synuclein Aggregation Pathway in Parkinson’s Disease: Current Status and Novel Therapeutic Approaches. Cells 2022, 11, 1732. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Pike, A.F.; Varanita, T.; Herrebout, M.A.; Plug, B.C.; Kole, J.; Musters, R.J.; Teunissen, C.E.; Hoozemans, J.J.; Bubacco, L.; Veerhuis, R. α-Synuclein evokes NLRP3 inflammasome-mediated IL-1β secretion from primary human microglia. Glia 2021, 69, 1413–1428. [Google Scholar] [CrossRef]
- Soraci, L.; Gambuzza, M.E.; Biscetti, L.; Laganà, P.; Lo Russo, C.; Buda, A.; Barresi, G.; Corsonello, A.; Lattanzio, F.; Lorello, G. Toll-like receptors and NLRP3 inflammasome-dependent pathways in Parkinson’s disease: Mechanisms and therapeutic implications. J. Neurol. 2023, 270, 1346–1360. [Google Scholar] [CrossRef]
- Heidari, A.; Yazdanpanah, N.; Rezaei, N. The role of Toll-like receptors and neuroinflammation in Parkinson’s disease. J. Neuroinflamm. 2022, 19, 135. [Google Scholar] [CrossRef]
- Szabo, A.; O‘Connell, K.S.; Ueland, T.; Sheikh, M.A.; Agartz, I.; Andreou, D.; Aukrust, P.; Boye, B.; Bøen, E.; Drange, O.K. Increased circulating IL-18 levels in severe mental disorders indicate systemic inflammasome activation. Brain Behav. Immun. 2022, 99, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Alboni, S.; Tascedda, F.; Uezato, A.; Sugama, S.; Chen, Z.; Marcondes, M.C.G.; Conti, B. Interleukin 18 and the brain: Neuronal functions, neuronal survival and psycho-neuro-immunology during stress. Mol. Psychiatry 2025, 30, 3197–3208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Shi, L.; Wang, Y.; Chen, S.; Zhang, J. Recent advances of the NLRP3 inflammasome in central nervous system disorders. J. Immunol. Res. 2016, 2016, 9238290. [Google Scholar] [CrossRef] [PubMed]
- von Herrmann, K.M.; Salas, L.A.; Martinez, E.M.; Young, A.L.; Howard, J.M.; Feldman, M.S.; Christensen, B.C.; Wilkins, O.M.; Lee, S.L.; Hickey, W.F.; et al. NLRP3 expression in mesencephalic neurons and characterization of a rare NLRP3 polymorphism associated with decreased risk of Parkinson’s disease. NPJ Park. Dis. 2018, 4, 24. [Google Scholar] [CrossRef]
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 2019, 10, 3945, Erratum in Nat. Commun. 2019, 10, 4725. [Google Scholar] [CrossRef]
- Shen, X.; Venero, J.L.; Joseph, B.; Burguillos, M.A. Caspases orchestrate microglia instrumental functions. Prog. Neurobiol. 2018, 171, 50–71. [Google Scholar] [CrossRef]
- Qiao, C.; Zhang, L.X.; Sun, X.Y.; Ding, J.H.; Lu, M.; Hu, G. Caspase-1 Deficiency Alleviates Dopaminergic Neuronal Death via Inhibiting Caspase-7/AIF Pathway in MPTP/p Mouse Model of Parkinson’s Disease. Mol. Neurobiol. 2017, 54, 4292–4302. [Google Scholar] [CrossRef]
- Park, J.K.; Doseff, A.I.; Schmittgen, T.D. MicroRNAs Targeting Caspase-3 and -7 in PANC-1 Cells. Int. J. Mol. Sci. 2018, 19, 1206. [Google Scholar] [CrossRef]
- Li, D.; Yang, H.; Ma, J.; Luo, S.; Chen, S.; Gu, Q. MicroRNA-30e regulates neuroinflammation in MPTP model of Parkinson’s disease by targeting Nlrp3. Hum. Cell 2018, 31, 106–115. [Google Scholar] [CrossRef]
- Henrich, M.T.; Oertel, W.H.; Surmeier, D.J.; Geibl, F.F. Mitochondrial dysfunction in Parkinson’s disease—A key disease hallmark with therapeutic potential. Mol. Neurodegener. 2023, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, Z.; Han, X.; Wang, D.; Jiang, Q.; Ding, J.; Xiao, M.; Wang, C.; Lu, M.; Hu, G. Dopamine D2 receptor restricts astrocytic NLRP3 inflammasome activation via enhancing the interaction of β-arrestin2 and NLRP3. Cell Death Differ. 2018, 25, 2037–2049. [Google Scholar] [CrossRef]
- Wu, F.; Xu, H.-D.; Guan, J.-J.; Hou, Y.-S.; Gu, J.-H.; Zhen, X.-C.; Qin, Z.-H. Rotenone impairs autophagic flux and lysosomal functions in Parkinson’s disease. Neuroscience 2015, 284, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.M.; Young, A.L.; Patankar, Y.R.; Berwin, B.L.; Wang, L.; von Herrmann, K.M.; Weier, J.M.; Havrda, M.C. Editor’s Highlight: Nlrp3 Is Required for Inflammatory Changes and Nigral Cell Loss Resulting from Chronic Intragastric Rotenone Exposure in Mice. Toxicol. Sci. Off. J. Soc. Toxicol. 2017, 159, 64–75. [Google Scholar] [CrossRef]
- El-Gamal, M.; Salama, M.; Collins-Praino, L.E.; Baetu, I.; Fathalla, A.M.; Soliman, A.M.; Mohamed, W.; Moustafa, A.A. Neurotoxin-induced rodent models of Parkinson’s disease: Benefits and drawbacks. Neurotox. Res. 2021, 39, 897–923. [Google Scholar] [CrossRef]
- Altunlu, Ö.; Topatan, E.; Al-yaqoobi, Z.; Burul, F.; Bayram, C.; Sezen, S.; Okkay, I.F.; Okkay, U.; Hacımüftüoğlu, A. Experimental Models in Parkinson’s Disease: Advantages and Disadvantages. Ağrı Tıp Fakültesi Derg. 2024, 2, 80–87. [Google Scholar] [CrossRef]
- Qian, L.; Tcw, J. Human iPSC-based modeling of central nerve system disorders for drug discovery. Int. J. Mol. Sci. 2021, 22, 1203. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, W.; Zhang, Z.; Ai, X.; Cao, B.; Zhang, Z.; Ma, D. Induced Pluripotent Stem Cells Derived Cellular Models for Investigating Parkinson’s Disease Pathogenesis and Drug Screening. Stem Cell Rev. Rep. 2025, 21, 1883–1900. [Google Scholar] [CrossRef]
- De Gaetano, A.; Solodka, K.; Zanini, G.; Selleri, V.; Mattioli, A.V.; Nasi, M.; Pinti, M. Molecular mechanisms of mtDNA-mediated inflammation. Cells 2021, 10, 2898. [Google Scholar] [CrossRef]
- Kwatra, M.; Ahmed, S.; Gangipangi, V.K.; Panda, S.R.; Gupta, N.; Shantanu, P.; Gawali, B.; Naidu, V. Lipopolysaccharide exacerbates chronic restraint stress-induced neurobehavioral deficits: Mechanisms by redox imbalance, ASK1-related apoptosis, autophagic dysregulation. J. Psychiatr. Res. 2021, 144, 462–482. [Google Scholar] [CrossRef]
- Hou, X.; Watzlawik, J.O.; Fiesel, F.C.; Springer, W. Autophagy in Parkinson’s disease. J. Mol. Biol. 2020, 432, 2651–2672. [Google Scholar] [CrossRef] [PubMed]
- Minchev, D.; Kazakova, M.; Sarafian, V. Neuroinflammation and autophagy in Parkinson’s disease—Novel perspectives. Int. J. Mol. Sci. 2022, 23, 14997. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Debnath, J. Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 2015, 16, 461–472. [Google Scholar] [CrossRef]
- Yamamoto, H.; Matsui, T. Molecular mechanisms of macroautophagy, microautophagy, and chaperone-mediated autophagy. J. Nippon Med. Sch. 2024, 91, 2–9. [Google Scholar] [CrossRef]
- Yim, W.W.-Y.; Mizushima, N. Lysosome biology in autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef]
- Ke, P.-Y. Molecular mechanism of autophagosome–lysosome fusion in mammalian cells. Cells 2024, 13, 500. [Google Scholar] [CrossRef] [PubMed]
- Cen, X.; Li, Z.; Chen, X. Ubiquitination in the regulation of autophagy: Ubiquitination in the regulation of autophagy. Acta Biochim. Biophys. Sin. 2023, 55, 1348. [Google Scholar] [CrossRef]
- Nixon, R.A. Autophagy–lysosomal-associated neuronal death in neurodegenerative disease. Acta Neuropathol. 2024, 148, 42. [Google Scholar] [CrossRef] [PubMed]
- Valionyte, E. Characterisation of Caspase-6-Dependent Regulatory Mechanisms of SQSTM1/p62 Droplet-Based Autophagy; University of Plymouth: Plymouth, UK, 2023. [Google Scholar]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2023, 19, 388–400. [Google Scholar] [CrossRef]
- Malpartida, A.B.; Williamson, M.; Narendra, D.P.; Wade-Martins, R.; Ryan, B.J. Mitochondrial dysfunction and mitophagy in Parkinson’s disease: From mechanism to therapy. Trends Biochem. Sci. 2021, 46, 329–343. [Google Scholar] [CrossRef]
- Singh, F.; Ganley, I.G. Parkinson’s disease and mitophagy: An emerging role for LRRK2. Biochem. Soc. Trans. 2021, 49, 551–562. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Lee, E.; Hwang, I.; Park, S.; Hong, S.; Hwang, B.; Cho, Y.; Son, J.; Yu, J.-W. MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ. 2019, 26, 213–228. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Gupta, S.; Cassel, S.L.; Sutterwala, F.S.; Dagvadorj, J. Regulation of the NLRP3 inflammasome by autophagy and mitophagy. Immunol. Rev. 2025, 329, e13410. [Google Scholar] [CrossRef] [PubMed]
- Lackner, A.; Leonidas, L.; Macapagal, A.; Lee, H.; McNulty, R. How interactions between oxidized DNA and the NLRP3 inflammasome fuel inflammatory disease. Trends Biochem. Sci. 2025, 50, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.W.; Ordureau, A.; Heo, J.-M. Building and decoding ubiquitin chains for mitophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 93–108. [Google Scholar] [CrossRef]
- Gadhave, K.; Bolshette, N.; Ahire, A.; Pardeshi, R.; Thakur, K.; Trandafir, C.; Istrate, A.; Ahmed, S.; Lahkar, M.; Muresanu, D.F. The ubiquitin proteasomal system: A potential target for the management of Alzheimer’s disease. J. Cell. Mol. Med. 2016, 20, 1392–1407. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Li, R.; Yang, H. Mitophagy in Parkinson’s disease: From pathogenesis to treatment. Cells 2019, 8, 712. [Google Scholar] [CrossRef]
- Dernie, F. Mitophagy in Parkinson’s disease: From pathogenesis to treatment target. Neurochem. Int. 2020, 138, 104756. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Holzbaur, E.L. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc. Natl. Acad. Sci. USA 2014, 111, E4439–E4448. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Fiesel, F.C.; Truban, D.; Castanedes Casey, M.; Lin, W.-l.; Soto, A.I.; Tacik, P.; Rousseau, L.G.; Diehl, N.N.; Heckman, M.G. Age-and disease-dependent increase of the mitophagy marker phospho-ubiquitin in normal aging and Lewy body disease. Autophagy 2018, 14, 1404–1418. [Google Scholar] [CrossRef]
- Clark, I.E.; Dodson, M.W.; Jiang, C.; Cao, J.H.; Huh, J.R.; Seol, J.H.; Yoo, S.J.; Hay, B.A.; Guo, M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 2006, 441, 1162–1166. [Google Scholar] [CrossRef]
- Morais, V.A.; Haddad, D.; Craessaerts, K.; De Bock, P.-J.; Swerts, J.; Vilain, S.; Aerts, L.; Overbergh, L.; Grünewald, A.; Seibler, P. PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science 2014, 344, 203–207. [Google Scholar] [CrossRef]
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 2004, 304, 1158–1160. [Google Scholar] [CrossRef]
- Chen, Y.; Dorn, G.W. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 2013, 340, 471–475. [Google Scholar] [CrossRef]
- Shi, R.Y.; Zhu, S.H.; Li, V.; Gibson, S.B.; Xu, X.S.; Kong, J.M. BNIP3 interacting with LC3 triggers excessive mitophagy in delayed neuronal death in stroke. CNS Neurosci. Ther. 2014, 20, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Feng, D.; Chen, G.; Chen, M.; Zheng, Q.; Song, P.; Ma, Q.; Zhu, C.; Wang, R.; Qi, W. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 2012, 14, 177–185. [Google Scholar] [CrossRef]
- He, Y.-L.; Li, J.; Gong, S.-H.; Cheng, X.; Zhao, M.; Cao, Y.; Zhao, T.; Zhao, Y.-Q.; Fan, M.; Wu, H.-T. BNIP3 phosphorylation by JNK1/2 promotes mitophagy via enhancing its stability under hypoxia. Cell Death Dis. 2022, 13, 966. [Google Scholar] [CrossRef]
- Lai, M.; Yao, H.; Shah, S.Z.A.; Wu, W.; Wang, D.; Zhao, Y.; Wang, L.; Zhou, X.; Zhao, D.; Yang, L. The NLRP3-Caspase 1 Inflammasome Negatively Regulates Autophagy via TLR4-TRIF in Prion Peptide-Infected Microglia. Front. Aging Neurosci. 2018, 10, 116. [Google Scholar] [CrossRef]
- Ahmed, S.; Kwatra, M.; Panda, S.R.; Murty, U.; Naidu, V. Andrographolide suppresses NLRP3 inflammasome activation in microglia through induction of parkin-mediated mitophagy in in-vitro and in-vivo models of Parkinson disease. Brain Behav. Immun. 2021, 91, 142–158. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, Y.; Zhuo, J.; Zhang, L.; Liu, J.; Wang, B.; Sun, D.; Yu, S.; Lou, H. Urolithin A promotes mitophagy and suppresses NLRP3 inflammasome activation in lipopolysaccharide-induced BV2 microglial cells and MPTP-induced Parkinson’s disease model. Neuropharmacology 2022, 207, 108963. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Lo, H.; Zheng, J.; Weng, W.; Sun, Y.; Pan, X. Cycloastragenol reduces microglial NLRP3 inflammasome activation in Parkinson’s disease models by promoting autophagy and reducing Scrib-driven ROS. Phytomedicine 2024, 135, 156210. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.-q.; Yang, Y.-p.; Jiang, S.-m.; Yao, X.-y.; Qi, D.; Mao, C.-j.; Cheng, X.-y.; Wang, F.; Hu, L.-f.; Liu, C.-f. Pramipexole inhibits astrocytic NLRP3 inflammasome activation via Drd3-dependent autophagy in a mouse model of Parkinson’s disease. Acta Pharmacol. Sin. 2023, 44, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, J.; Zhao, K.; Yang, S.; Dong, J.; Zhang, Y.; Wu, S.; Xiang, L.; Hu, W. Palmatine Ameliorates Motor Deficits and Dopaminergic Neuron Loss by Regulating NLRP3 Inflammasome through Mitophagy in Parkinson’s Disease Model Mice. Mol. Neurobiol. 2025, 62, 2250–2263. [Google Scholar] [CrossRef]
- Trudler, D.; Nazor, K.L.; Eisele, Y.S.; Grabauskas, T.; Dolatabadi, N.; Parker, J.; Sultan, A.; Zhong, Z.; Goodwin, M.S.; Levites, Y. Soluble α-synuclein–antibody complexes activate the NLRP3 inflammasome in hiPSC-derived microglia. Proc. Natl. Acad. Sci. USA 2021, 118, e2025847118. [Google Scholar] [CrossRef]
- Panicker, N.; Kam, T.-I.; Wang, H.; Neifert, S.; Chou, S.-C.; Kumar, M.; Brahmachari, S.; Jhaldiyal, A.; Hinkle, J.T.; Akkentli, F. Neuronal NLRP3 is a parkin substrate that drives neurodegeneration in Parkinson’s disease. Neuron 2022, 110, 2422–2437.e9. [Google Scholar] [CrossRef]
- Qin, Y.; Qiu, J.; Wang, P.; Liu, J.; Zhao, Y.; Jiang, F.; Lou, H. Impaired autophagy in microglia aggravates dopaminergic neurodegeneration by regulating NLRP3 inflammasome activation in experimental models of Parkinson’s disease. Brain Behav. Immun. 2021, 91, 324–338. [Google Scholar] [CrossRef]
- Chen, J.; Mao, K.; Yu, H.; Wen, Y.; She, H.; Zhang, H.; Liu, L.; Li, M.; Li, W.; Zou, F. p38-TFEB pathways promote microglia activation through inhibiting CMA-mediated NLRP3 degradation in Parkinson’s disease. J. Neuroinflamm. 2021, 18, 295. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Nixon, R.A. Rapamycin induces autophagic flux in neurons. Proc. Natl. Acad. Sci. USA 2010, 107, E181. [Google Scholar] [CrossRef] [PubMed]
- Demaré, S.; Kothari, A.; Calcutt, N.A.; Fernyhough, P. Metformin as a potential therapeutic for neurological disease: Mobilizing AMPK to repair the nervous system. Expert Rev. Neurother. 2021, 21, 45–63. [Google Scholar] [CrossRef]
- Rusmini, P.; Cortese, K.; Crippa, V.; Cristofani, R.; Cicardi, M.E.; Ferrari, V.; Vezzoli, G.; Tedesco, B.; Meroni, M.; Messi, E. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2019, 15, 631–651. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Stitham, J.; Evans, T.D.; Zhang, X.; Rodriguez-Velez, A.; Yeh, Y.-S.; Tao, J.; Takabatake, K.; Epelman, S.; Lodhi, I.J. Trehalose causes low-grade lysosomal stress to activate TFEB and the autophagy-lysosome biogenesis response. Autophagy 2021, 17, 3740–3752. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Sankhe, R.; Mudgal, J.; Arora, D.; Nampoothiri, M. Spermidine, an autophagy inducer, as a therapeutic strategy in neurological disorders. Neuropeptides 2020, 83, 102083. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Zhang, Y.; Lin, X.; Song, Y.; Xue, Z.; Qian, H.; Wang, S.; Wan, G.; Zheng, X. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis. 2017, 8, e2738. [Google Scholar] [CrossRef]
- Chang, Y.P.; Ka, S.M.; Hsu, W.H.; Chen, A.; Chao, L.K.; Lin, C.C.; Hsieh, C.C.; Chen, M.C.; Chiu, H.W.; Ho, C.L. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J. Cell. Physiol. 2015, 230, 1567–1579. [Google Scholar] [CrossRef]
- Biasizzo, M.; Kopitar-Jerala, N. Interplay between NLRP3 inflammasome and autophagy. Front. Immunol. 2020, 11, 591803. [Google Scholar] [CrossRef]
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Muñoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef]
- Sánchez-Fernández, A.; Skouras, D.B.; Dinarello, C.A.; López-Vales, R. OLT1177 (Dapansutrile), a selective NLRP3 inflammasome inhibitor, ameliorates experimental autoimmune encephalomyelitis pathogenesis. Front. Immunol. 2019, 10, 2578. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liang, C.; Huang, K.; Luo, J.; Lu, R.; Lai, Y.; Zheng, D.; Lin, Z.; Zhong, J.; Dai, J. Curcumin prevents neurodegeneration by blocking HDAC6–NLRP3 pathway-dependent neuroinflammation in Parkinson’s disease. Int. Immunopharmacol. 2025, 146, 113928. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Sun, S.; Sun, Y.; Song, Q.; Zhu, J.; Song, N.; Chen, M.; Sun, T.; Xia, M.; Ding, J. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: Implications for Parkinson disease. Autophagy 2019, 15, 1860–1881. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Li, R.; Zhu, L.; Fu, B.; Yan, T. Salidroside ameliorates Parkinson’s disease by inhibiting NLRP3-dependent pyroptosis. Aging 2020, 12, 9405. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mohapatra, J.; Sharma, M.; Jha, A.; Patro, R.; Das, D.; Patel, H.; Patel, H.; Chaudhari, J.; Borda, N. A novel selective NLRP3 inhibitor shows disease-modifying potential in animal models of Parkinson’s disease. Brain Res. 2024, 1842, 149129. [Google Scholar] [CrossRef] [PubMed]
- Rui, W.; Li, S.; Xiao, H.; Xiao, M.; Shi, J. Baicalein attenuates neuroinflammation by inhibiting NLRP3/caspase-1/GSDMD pathway in MPTP-induced mice model of Parkinson’s disease. Int. J. Neuropsychopharmacol. 2020, 23, 762–773. [Google Scholar] [CrossRef]
- Gao, M.-R.; Wang, M.; Jia, Y.-Y.; Tian, D.-D.; Liu, A.; Wang, W.-J.; Yang, L.; Chen, J.-Y.; Yang, Q.; Liu, R. Echinacoside protects dopaminergic neurons by inhibiting NLRP3/Caspase-1/IL-1β signaling pathway in MPTP-induced Parkinson’s disease model. Brain Res. Bull. 2020, 164, 55–64. [Google Scholar] [CrossRef]
- Yan, S.; Wei, X.; Jian, W.; Qin, Y.; Liu, J.; Zhu, S.; Jiang, F.; Lou, H.; Zhang, B. Pharmacological inhibition of HDAC6 attenuates NLRP3 inflammatory response and protects dopaminergic neurons in experimental models of Parkinson’s disease. Front. Aging Neurosci. 2020, 12, 78. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, Q.; Hou, L.; Zhang, C.; Wang, Q.; Zhao, X. Inhibition of NLRP3 inflammasome by glibenclamide attenuated dopaminergic neurodegeneration and motor deficits in paraquat and maneb-induced mouse Parkinson’s disease model. Toxicol. Lett. 2021, 349, 1–11. [Google Scholar] [CrossRef]
- Zhang, M.; He, Q.; Chen, G.; Li, P.A. Suppression of NLRP3 inflammasome, pyroptosis, and cell death by NIM811 in rotenone-exposed cells as an in vitro model of Parkinson’s disease. Neurodegener. Dis. 2020, 20, 73–83. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Gu, R.; Che, N.; Wang, J.; Cheng, J.; Yuan, Z.; Cheng, Y.; Liao, Y. The XPO1 inhibitor KPT-8602 ameliorates Parkinson’s disease by inhibiting the NF-κB/NLRP3 pathway. Front. Pharmacol. 2022, 13, 847605. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, W.-m.; Tang, P.-c.; Zhang, X.; Zhang, X.-y.; Yu, B.-c.; Fan, Y.-Y.; Ge, X.-q.; Zhang, X.-L. Neuroprotective effects of natural cordycepin on LPS-induced Parkinson’s disease through suppressing TLR4/NF-κB/NLRP3-mediated pyroptosis. J. Funct. Foods 2020, 75, 104274. [Google Scholar] [CrossRef]
- Jiang, H.; He, H.; Chen, Y.; Huang, W.; Cheng, J.; Ye, J.; Wang, A.; Tao, J.; Wang, C.; Liu, Q. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 2017, 214, 3219–3238. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, J.; Xu, Q.; Long, T.; Bi, F.; Chang, C.; Liu, W. Rapamycin improves the neuroprotection effect of inhibition of NLRP3 inflammasome activation after TBI. Brain Res. 2019, 1710, 163–172. [Google Scholar] [CrossRef]
- Li, A.; Zhang, S.; Li, J.; Liu, K.; Huang, F.; Liu, B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol. Cell. Endocrinol. 2016, 434, 36–47. [Google Scholar] [CrossRef]
- Ahmed, S.; Panda, S.R.; Kwatra, M.; Sahu, B.D.; Naidu, V.G.M. Perillyl Alcohol Attenuates NLRP3 Inflammasome Activation and Rescues Dopaminergic Neurons in Experimental In Vitro and In Vivo Models of Parkinson’s Disease. ACS Chem. Neurosci. 2022, 13, 53–68. [Google Scholar] [CrossRef]
- Clarke, N.; Thornton, P.; Reader, V.; Lindsay, N.; Digby, Z.; Mullen, B.; Gorman, M.; Jacobson, E.; Langdon, G.; Johnstone, H. Anti-Neuroinflammatory and Anti-Inflammatory Effects of the NLRP3 Inhibitor NT-0796 in Subjects with Parkinson’s Disease. Mov. Disord. 2025, 40, 2199–2208. [Google Scholar] [CrossRef]
- Kaur, B.; Biby, S.; Namme, J.N.; More, S.; Xu, Y.; Zhang, S. Biological and therapeutic significance of targeting NLRP3 inflammasome in the brain and the current efforts to develop brain-penetrant inhibitors. Adv. Pharmacol. 2025, 102, 103–157. [Google Scholar] [CrossRef]
- Luque, M.; Matic, M.; Heras-Garvin, A.; Amo-Aparicio, J.; Luk, K.; Haindl, M.T.; Khalil, M.; Skouras, D.B.; Dinarello, C.A.; Stefanova, N. Pharmacologic NLRP3 Inhibition Modulates Parkinson′s Disease-Associated Microglial Transcriptomic Signatures and Mitigates α-Synuclein-Triggered Neurodegeneration. bioRxiv 2025, 10, 683837. [Google Scholar]
- Philippidis, A. Insilico’s AI Workflow Has Two Jobs: Discover Compounds, Thwart Competitors: Company researchers unveil LEGION, designed to search chemical space around targets, then protect discoveries from fast patenting by rivals. GEN Edge 2025, 7, 636–639. [Google Scholar] [CrossRef]
- Bhansali, S.; Bhansali, A.; Dutta, P.; Walia, R.; Dhawan, V. Metformin upregulates mitophagy in patients with T2DM: A randomized placebo-controlled study. J. Cell. Mol. Med. 2020, 24, 2832–2846. [Google Scholar] [CrossRef] [PubMed]
- Gouda, N.A.; Elkamhawy, A.; Cho, J. Emerging Therapeutic Strategies for Parkinson’s Disease and Future Prospects: A 2021 Update. Biomedicines 2022, 10, 371. [Google Scholar] [CrossRef]
- Wojciechowska, O.; Kujawska, M. Urolithin A in Health and Diseases: Prospects for Parkinson’s Disease Management. Antioxidants 2023, 12, 1479. [Google Scholar] [CrossRef]
- Fang, T.-S.Z.; Sun, Y.; Pearce, A.C.; Eleuteri, S.; Kemp, M.; Luckhurst, C.A.; Williams, R.; Mills, R.; Almond, S.; Burzynski, L. Knockout or inhibition of USP30 protects dopaminergic neurons in a Parkinson’s disease mouse model. Nat. Commun. 2023, 14, 7295. [Google Scholar] [CrossRef]
- Bordt, E.A.; Polster, B.M. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: A bipartisan affair? Free Radic. Biol. Med. 2014, 76, 34–46. [Google Scholar] [CrossRef]
- Shao, B.-Z.; Xu, Z.-Q.; Han, B.-Z.; Su, D.-F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Sadier, N.S.; Hazimeh, I.A.; Khazaal, W.; Al Sabouri, A.A.K.; Almutary, A.G.; Alnuqaydan, A.M.; Abou-Abbas, L. Exploring the therapeutic potential of NLRP3 inhibitors in Parkinson’s Disease: A systematic review of in-vivo studies. Inflammopharmacology 2025, 33, 2657–2677. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Hu, Z.; Zhong, Y.; Wu, C.; Li, J. The potential of NLRP3 inflammasome as a therapeutic target in neurological diseases. Mol. Neurobiol. 2023, 60, 2520–2538. [Google Scholar] [CrossRef] [PubMed]
- Pinzón-Fernández, M.V.; Saavedra-Torres, J.S.; López Garzón, N.A.; Pachón Bueno, J.S.; Tamayo-Giraldo, F.J.; Rojas Gomez, M.C.; Arias-Intriago, M.; Gaibor-Pazmiño, A.; López-Cortés, A.; Izquierdo-Condoy, J.S. NLRP3 and beyond: Inflammasomes as central cellular hub and emerging therapeutic target in inflammation and disease. Front. Immunol. 2025, 16, 1624770. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef]
- Moors, T.E.; Hoozemans, J.J.; Ingrassia, A.; Beccari, T.; Parnetti, L.; Chartier-Harlin, M.-C.; Van De Berg, W.D. Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol. Neurodegener. 2017, 12, 11. [Google Scholar] [CrossRef]
- Fowler, A.J.; Moussa, C.E.-H. Activating autophagy as a therapeutic strategy for Parkinson’s disease. CNS Drugs 2018, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fivenson, E.M.; Lautrup, S.; Sun, N.; Scheibye-Knudsen, M.; Stevnsner, T.; Nilsen, H.; Bohr, V.A.; Fang, E.F. Mitophagy in neurodegeneration and aging. Neurochem. Int. 2017, 109, 202–209. [Google Scholar] [CrossRef]
- Twig, G.; Shirihai, O.S. The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef]
- Carter, F.E.; Moore, M.E.; Pickrell, A.M. Methods to detect mitophagy in neurons during disease. J. Neurosci. Methods 2019, 325, 108351. [Google Scholar] [CrossRef]
- Ashrafi, G.; Schwarz, T. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef]
- Montava-Garriga, L.; Ganley, I.G. Outstanding questions in mitophagy: What we do and do not know. J. Mol. Biol. 2020, 432, 206–230. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.J.; Hoek, S.; Fon, E.A.; Wade-Martins, R. Mitochondrial dysfunction and mitophagy in Parkinson’s: From familial to sporadic disease. Trends Biochem. Sci. 2015, 40, 200–210. [Google Scholar] [CrossRef]
- Zhu, R.-X.; Han, R.-X.; Chen, Y.-H.; Huang, L.; Liu, T.; Jiang, J.; Wang, C.; Cao, L.; Liu, Y.; Lu, M. Inactivation of NLRP3 inflammasome by dephosphorylation at Serine 658 alleviates glial inflammation in the mouse model of Parkinson’s disease. Mol. Neurodegener. 2025, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Kodi, T.; Sankhe, R.; Gopinathan, A.; Nandakumar, K.; Kishore, A. New Insights on NLRP3 Inflammasome: Mechanisms of Activation, Inhibition, and Epigenetic Regulation. J. Neuroimmune Pharmacol. 2024, 19, 7. [Google Scholar] [CrossRef]
- Therriault, J.; Schindler, S.E.; Salvadó, G.; Pascoal, T.A.; Benedet, A.L.; Ashton, N.J.; Karikari, T.K.; Apostolova, L.; Murray, M.E.; Verberk, I.; et al. Biomarker-based staging of Alzheimer disease: Rationale and clinical applications. Nat. Rev. Neurol. 2024, 20, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Lowes, H.; Pyle, A.; Santibanez-Koref, M.; Hudson, G. Circulating cell-free mitochondrial DNA levels in Parkinson’s disease are influenced by treatment. Mol. Neurodegener. 2020, 15, 10. [Google Scholar] [CrossRef]
- Su, Q.; Ng, W.L.; Goh, S.Y.; Gulam, M.Y.; Wang, L.F.; Tan, E.K.; Ahn, M.; Chao, Y.X. Targeting the inflammasome in Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 957705. [Google Scholar] [CrossRef] [PubMed]
| Compound | Mechanistic Evidence (from In Vitro/In Vivo Studies) | Developmental Stage | Major Limitation/Cause of Failure | Reference |
|---|---|---|---|---|
| Rapamycin (Sirolimus) | In vitro: Primary neurons. Rapamycin increases autophagic flux by enhancing autophagosome formation and autophagosome–lysosome fusion, evidenced by LC3-II turnover. In vivo: Not reported in this study. | Preclinical (PD models) | Immunosuppressant; metabolic side effect; narrow therapeutic window; poor BBB penetration | [134] |
| Metformin | In vitro (adult DRG sensory neurons): Metformin activates AMPK; suppresses mitochondrial electron transport; promotes neurite outgrowth; proposed to upregulate autophagy via AMPK → likely mTORC1 suppression. In vivo (sciatic nerve injury model): Metformin increases LC3-II (autophagy marker), enhances autophagy; correlates with reduced cell death, improved myelination and motor recovery. Autophagy inhibition abolishes these benefits. | Preclinical (PD-related models); epidemiological studies | Conflicting clinical outcome; metabolic side effect | [135] |
| Trehalose | NSC-34 and primary motoneurons. Trehalose activates TFEB via lysosomal mechanisms, increasing autophagy and lysosomal biogenesis; reduces misfolded protein accumulation. trehalose induces low-grade lysosomal stress → TFEB activation → enhanced autophagy–lysosome gene expression and lysosomal expansion. In vivo: In mice, trehalose induces TFEB activation in liver tissue, increasing autophagy–lysosome biogenesis. | Preclinical | Poor bioavailability; rapid degradation by trehalase; limited BBB penetration | [136,137] |
| Spermidine | In vitro: primary neuron-spermidine induces autophagy by preventing caspase-3–mediated cleavage of Beclin-1, preserving autophagy initiation; increases autophagic flux and protects against apoptosis. Summarizes evidence that spermidine activates autophagy through an mTOR-independent pathway in various in vitro models (cell types not specified in the review). In vivo: Spermidine enhances neuronal autophagy, reduces caspase-3 activity, preserves Beclin-1, and provides neuroprotection. Summarizes that spermidine has autophagy-dependent neuroprotective and anti-aging effects in multiple in vivo models. | Preclinical | Dose optimization unclear; long-term safety in CNS not established | [138,139] |
| Resveratrol | BMDMs and THP-1 cells. Resveratrol activates autophagy, preserves mitochondrial integrity, and inhibits NLRP3 inflammasome (↓ caspase-1 cleavage, ↓ IL-1β); autophagy is required for this effect. Summarizes that resveratrol activates SIRT1/AMPK → autophagy and inhibits NLRP3 inflammasome in immune cells. In vivo: Mouse model. Resveratrol increases autophagy in splenocytes and reduces NLRP3 inflammasome activation; confirms autophagy-dependent anti-inflammatory effects. Summarizes in vivo data from prior studies supporting autophagy-dependent inhibition of NLRP3 by resveratrol. | Preclinical; limited clinical studies (non-PD) | Poor stability and bioavailability; low BBB penetration | [140,141] |
| Compound | Mechanistic Evidence (In Vitro/In Vivo) | Developmental Stage | Major Limitation/Cause of Failure | Reference |
|---|---|---|---|---|
| MCC950 | In vitro: Selectively inhibits NLRP3 by blocking NACHT ATPase activity → prevents NLRP3 oligomerization, ASC speck formation, and IL-1β/IL-18 release in macrophages; no effect on AIM2 or NLRC4 inflammasomes. In vivo: Reduces IL-1β levels and inflammation in NLRP3-driven mouse models (LPS septic shock, MWS/CAPS models, EAE), improving survival and clinical symptoms via selective NLRP3 inhibition. | Terminated in Phase II trial | Hepatotoxicity; long-term safety concern | [142] |
| Dapansutrile | In vivo: In EAE mice, it reduces IL-1β, IL-18, IL-6, and TNFα in spinal cord; attenuates immune cell infiltration and demyelination, improving clinical scores, consistent with selective NLRP3 inflammasome inhibition. In vitro: Not reported. | Phase II/III trail | Limited CNS efficacy data | [143] |
| β-Hydroxybutyrate (BHB) | In vitro (macrophages): BHB selectively inhibits NLRP3 by blocking K+ efflux, preventing ASC oligomerization, caspase-1 activation, and IL-1β /IL-18 production. In vivo (mouse models): BHB suppresses NLRP3-driven inflammation in gout, LPS-induced inflammation, and MWS models, reducing IL-1β levels and neutrophil recruitment. | Pre-clinical | Limited specificity; BBB penetration unclear | [144] |
| Curcumin | In vitro (neuronal and inflammatory cell models): Curcumin inhibits the HDAC6–NLRP3 pathway, reducing NLRP3 activation and inflammatory signaling. In vivo (PD mouse model): Curcumin suppresses HDAC6-dependent NLRP3 activation, decreases neuroinflammation, and protects dopaminergic neurons. | Pre-clinical | Poor bioavailability; limited BBB penetration | [144,145] |
| Minocyclin | Suppresses microglial activation; inhibits NF-κB priming and NLRP3 activation. Reduces neuroinflammation and dopaminergic neuron loss in MPTP and 6-OHDA models. | Pre-clinical | Long-term toxicity concern | [146] |
| Salidroside | In vitro (PC-12/BV2 cells): Salidroside inhibits NLRP3-dependent pyroptosis by suppressing TLR4/NF-κB and TXNIP/NLRP3/caspase-1 signaling. In vivo (MPTP-PD mice): Reduces NLRP3 activation, IL-1β/IL-18, and GSDMD cleavage; protects dopaminergic neurons and improves PD symptoms. | Pre-clinical | No clinical trial data available; Pharmacokinetics unclear | [147] |
| Usnoflast (ZYIL1) | In vitro: ZYIL1 inhibits NLRP3 activation and IL-1β release in THP-1 cells, PBMCs, and microglia. In vivo: Reduces NLRP3 activation and IL-1β, protects dopaminergic neurons, and improves motor deficits in PD mouse models. | Pre-clinical | No clinical data; long-term safety data unknown | [148] |
| Baicalein | In vitro (glial cells): Baicalein inhibits NLRP3, caspase-1, IL-1β, and GSDMD cleavage, reducing pyroptosis. In vivo (MPTP-PD mice): Suppresses NLRP3/caspase-1/GSDMD pathway, decreases inflammation, protects dopaminergic neurons, and improves motor function. | Pre-clinical | Limited specificity; lack of clinical validation | [149] |
| Echinacoside | In vivo (MPTP-PD mice): Echinacoside inhibits NLRP3/caspase-1/IL-1β signaling, protects dopaminergic neurons, and improves motor behavior. In vitro: Not reported. | Pre-clinical | No invitro mechanistic data available | [150] |
| Tubastatin A | In vitro (SH-SY5Y cells): Tubastatin A inhibits NLRP3, caspase-1, and IL-1β activation. In vivo (6-OHDA PD mice): Reduces NLRP3-mediated inflammation, protects dopaminergic neurons, and improves nigrostriatal integrity. | Pre-clinical | Lack of safety concern; off-target effect seen | [151] |
| Dl-3-n-Butylphthalide | In vitro/In vivo: This study does not report experiments or mechanistic data for DL-3-n-Butylphthalide. The referenced paper examines Tubastatin A (HDAC6 inhibition) rather than NBP. | Not validated | No direct NLRP3 mechanistic evidence | [151] |
| Glibenclamide | In vitro (BV2 cells): Inhibits NLRP3, caspase-1, and IL-1β. In vivo (paraquat + PD mice): Reduces NLRP3 activation, protects dopaminergic neurons, and improves motor function. | Pre-clinical | Systemic hypoglycemia risk; off target effects | [152] |
| Cyclosporine A | In vitro (HT22 cells): NIM811 (CsA derivative) inhibits NLRP3, caspase-1, GSDMD, IL-1β/IL-18, and pyroptosis. In vivo: Not reported. | Pre-clinical | Immunosuppression; no in vivo PD validation | [153] |
| KPT-8602 | In vitro (BV2 cells): Inhibits NF-κB, NLRP3, caspase-1, and IL-1β. In vivo (PD mice): Suppresses NF-κB/NLRP3 signaling, reduces neuroinflammation, and protects dopaminergic neurons. | Pre-clinical | No clinical data | [154] |
| Cordycepin | In vitro (BV2 cells): Inhibits TLR4/NF-κB, NLRP3, caspase-1, and IL-1β. In vivo (LPS-PD mice): Suppresses TLR4/NF-κB/NLRP3 signaling and protects dopaminergic neurons. | Pre-clinical | Bioavailability and safety concern | [155] |
| CY-09 | In vitro/in vivo: No direct data for CY-09; the study focuses on p38-TFEB regulation of NLRP3 in microglia. | Mechanistic data only | No direct NLRP3 inhibition data | [133] |
| Oridonin | In vitro: Blocks NEK7–NLRP3 interaction, preventing caspase-1 activation and IL-1β/IL-18 release in macrophages. In vivo: Reduces NLRP3-mediated inflammation in mouse models of peritonitis, gout, and type 2 diabetes. | Pre-clinical | Toxicity concern; no PD-specific studies | [156] |
| Combination | Mechanistic Rationale | Mechanistic Evidence (In Vitro/In Vivo) | References |
|---|---|---|---|
| Rapamycin + MCC950 | Activates autophagy (Rapamycin) and inhibits NLRP3 inflammasome (MCC950) → synergistic neuroprotection. | In vitro (cortical neurons): Rapamycin activates autophagy; MCC950 inhibits NLRP3, reducing caspase-1 and IL-1β/IL-18. In vivo (TBI mice): Combination enhances neuroprotection, suppresses NLRP3 activation, and reduces neuronal damage. | [157] |
| Metformin + Resveratrol | Activates AMPK/SIRT1 → promotes autophagy and suppresses NLRP3 simultaneously. | In vitro (3T3-L1 cells): Activates AMPK, inhibits Drp1-mediated mitochondrial fission, ER stress, and NLRP3. In vivo (diabetic mice): Increases AMPK, reduces ROS, mitochondrial fission, ER stress, and NLRP3 activation. | [158] |
| Trehalose + β-Hydroxybutyrate | Trehalose promotes TFEB-mediated lysosomal/autophagy function; BHB inhibits NLRP3 → combined clearance and anti-inflammatory effect. | In vitro: BHB blocks NLRP3 activation in macrophages. In vivo: Reduces NLRP3-dependent IL-1β and inflammation in mice. | [144] |
| Kaempferol | Inhibit NLRP3 inflammasome activation and promote autophagy. | In vitro: Inhibits NLRP3 and IL-1β; promotes autophagy in BV2 cells. In vivo: Enhances autophagy, suppresses NLRP3, and protects dopaminergic neurons. | [146] |
| Andrographolide | Inhibit NLRP3 inflammasome activation and promote mitophagy. | In vitro: Induces parkin-mediated mitophagy; inhibits NLRP3 and IL-1β in microglia. In vivo: Enhances mitophagy, suppresses NLRP3, and protects dopaminergic neurons. | [125] |
| Perillyl Alcohol | Inhibit NLRP3 inflammasome activation by scavenging ROS production. | In vitro (microglia): Scavenges ROS, inhibits NLRP3 activation, and reduces IL-1β release. In vivo: Reduces ROS and NLRP3 inflammasome activation, protecting dopaminergic neurons. | [159] |
| Compound | Targets | Developmental Stage | Randomized | Treatment Duration | Therapeutic Potential, Safety, Efficacy | Outcomes/Key Finding | References/Patents/Trial |
|---|---|---|---|---|---|---|---|
| NT-0796 (Nod Thera) | NLRP3 inflammasome inhibitor | Phase Ib/2a trial completed | No | 28 days, with measurable effects as early as 7 days | Selective, brain-penetrant NLRP3 inhibitor showing anti-neuroinflammatory activity in PD; potential immunosuppression risk. | Safe, target engagement, biomarker reduction (NfL, sTREM2 in CSF); No direct data on α-synuclein. Next trail planned. | [160] |
| VTX3232-(Ventyx biosciences) | NLRP3 inhibitor | Phase 2a | No | 28 days daily oral dosing (40 mg) | Brain-penetrant, orally available NLRP3 inhibitor modulating microglial-driven neuroinflammation potential immune-related risks, long-term CNS safety untested. | Safe, reduced inflammation. No direct data on α-synuclein. Not failed. | [161], NCT06556173 |
| Dapansutrile (Olatec) (OLT1177) | NLRP3 inhibitor | Phase II “DAPA-PD” | Not yet conducted | Design details unpublished as of 2025 | Oral NLRP3 inhibitor modulating microglial activity and reducing α-synuclein neurodegeneration; well-tolerated with high safety, suitable for early or inflammation-driven PD. | Preclinical work suggests possible relevance, but no clinical PD data. | [162] |
| ISM8969 | N/A | Complete IND-enabling studies | N/A | N/A | Preclinical compound with completed IND-enabling studies; mechanism not disclosed. Safety, efficacy, and clinical potential remain untested, positioned for early-stage development. | Motor benefits in mice. Positive α-synuclein effects preclinically. | [163] |
| Metformin | AMPK activator, improve mitophagy | No PD RCTs’ observational only | Years (observational) | N/A | AMPK activator enhancing mitophagy in humans; well-tolerated, limited PD-modifying effect, potential adjunct or preventive therapy. | Mixed findings, no clear protective benefits, Preclinically have some effect on α-synuclein. | [164] |
| Rapamycin (Sirolimus) | mTOR inhibitor | Pre-clinical complete/No PD trials | N/A | N/A | mTOR inhibitor inducing autophagy with preclinical neuroprotective effects in PD models; significant immunosuppression limits long-term use, high disease-modifying potential but translational risk remains. | Preclinical data showed neuroprotective. | [165] |
| Urolithin A | Mitophagy inducer | N/A | N/A | N/A | Mitophagy inducer that promotes mitochondrial quality control; well-tolerated with minimal safety concerns, modest disease-modifying potential, suitable for preventive or adjunctive use in PD | No data clinically (Theoretical). | [166] |
| MTX325 (Mission Therapeutics) | USP30 inhibitor (mitophagy enhancer) | Phase I | N/A | N/A | USP30 inhibitor enhancing mitophagy and protecting dopaminergic neurons in PD models; early-phase clinical testing, safety and efficacy in humans not yet established, promising disease-modifying potential. | N/A | [167] |
| ABBV-1088 | PINK1 activator | Phase I (NCT06414798/NCT06579300) | Early phase data | No-placebo-controlled PD efficacy trials reported as of now (2025) | PINK1 activator targeting mitophagy in neurodegenerative disease. | Phase 1 results published mainly pharmacokinetics/safety in healthy subjects | [37], WO2021168446A1 |
| VNA-318 | Mitophagy activator | Phase I (NCT06721091) | Early trial | No-placebo-controlled PD efficacy trials reported as of now (2025) | Mitophagy activator in Phase I trials; safety and efficacy in humans untested, potential disease-modifying agent for PD pending clinical validation. | N/A | [37], US20220105117A1 |
| Selnoflast/Inflazome (Roche) | NLRP3 inflammasome inhibitor | Phase 1b with safety and tolerability in patients with early idiopathic PD | N/A | Not yet randomized | Brain-penetrant NLRP3 inhibitor showing safety and tolerability in early idiopathic PD; potential immune-related risks exist. Comparable efficacy to other NLRP3 inhibitors, with promise for early or inflammation-driven PD. | Data has not been released | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ahmed, S.; Pasam, T.; Afreen, F. Mitophagy–NLRP3 Inflammasome Crosstalk in Parkinson’s Disease: Pathogenic Mechanisms and Emerging Therapeutic Strategies. Int. J. Mol. Sci. 2026, 27, 486. https://doi.org/10.3390/ijms27010486
Ahmed S, Pasam T, Afreen F. Mitophagy–NLRP3 Inflammasome Crosstalk in Parkinson’s Disease: Pathogenic Mechanisms and Emerging Therapeutic Strategies. International Journal of Molecular Sciences. 2026; 27(1):486. https://doi.org/10.3390/ijms27010486
Chicago/Turabian StyleAhmed, Sahabuddin, Tulasi Pasam, and Farzana Afreen. 2026. "Mitophagy–NLRP3 Inflammasome Crosstalk in Parkinson’s Disease: Pathogenic Mechanisms and Emerging Therapeutic Strategies" International Journal of Molecular Sciences 27, no. 1: 486. https://doi.org/10.3390/ijms27010486
APA StyleAhmed, S., Pasam, T., & Afreen, F. (2026). Mitophagy–NLRP3 Inflammasome Crosstalk in Parkinson’s Disease: Pathogenic Mechanisms and Emerging Therapeutic Strategies. International Journal of Molecular Sciences, 27(1), 486. https://doi.org/10.3390/ijms27010486






