Emerging Insights into the Role of the Microbiome in Brain Gliomas: A Systematic Review of Recent Evidence
Abstract
1. Introduction
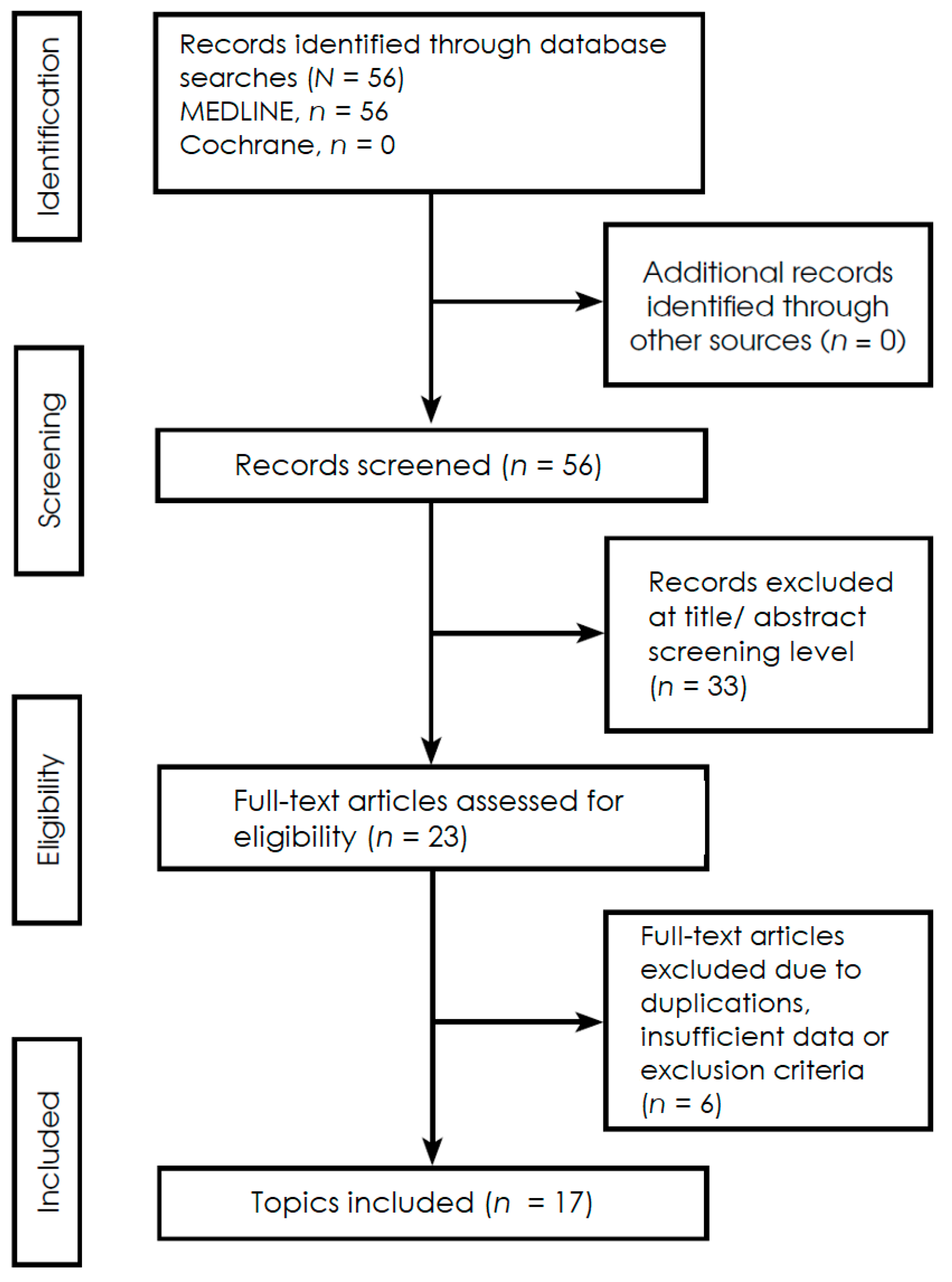
2. Methods
2.1. Protocol, Reporting Framework, Research Question, and Eligibility Criteria
2.2. Risk of Bias and Quality Appraisal
2.3. Review and Theoretical Frameworks
2.4. Multi-Modal Mechanistic Studies
2.5. Murine Model Studies
2.6. Cross-Species Research Projects
2.7. Therapy-Modulating Potential in Preclinical Models
2.8. Human Cohort Studies
2.9. Human Mendelian Randomization Studies
3. Results
3.1. Recurrent Microbial Signatures and Metabolic Pathways
3.2. Translational Relevance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Registration and Protocol
Abbreviations
| BBB | blood–brain barrier |
| CMV | human cytomegalovirus () |
| CNS | central nervous system |
| ENS | enteric nervous system |
| FDR | False Discovery Rate |
| GABA | gamma-aminobutyric acid |
| GALT | gut-associated lymphoid tissue |
| GAMs | glioma-associated macrophages/microglia |
| GBA | gut–brain axis |
| GBM | glioblastoma multiforme |
| GPCRs | G protein-coupled receptors |
| HGG | high-grade gliomas (WHO grades III–IV) |
| HDAC | histone deacetylase |
| HPA | hypothalamic–pituitary–adrenal axis |
| IDH1 | isocitrate dehydrogenase 1 |
| IHC | immunohistochemistry |
| IVW | inverse variance weighting |
| LGG | low-grade gliomas (WHO grades I–II) |
| MCTs | monocarboxylate transporters |
| MDSCs | myeloid-derived suppressor cells |
| MR | Mendelian randomization |
| NK | natural killer cells |
| PBS | phosphate-buffered saline |
| SCFAs | short-chain fatty acids |
| TAMs | tumor-associated macrophages |
| TCA | tricarboxylic acid |
| TCCs | T cell clones |
| TILs | tumor-infiltrating lymphocytes |
| TMZ | temozolomide |
| Tregs | regulatory T cells |
| WHO | World Health Organization |
References
- Hamad, A.; Yusubalieva, G.M.; Baklaushev, V.P.; Chumakov, P.M.; Lipatova, A.V. Recent Developments in Glioblastoma Therapy: Oncolytic Viruses and Emerging Future Strategies. Viruses 2023, 15, 547. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.H.; La Fata, G.; Steinert, R.E.; Weber, P. Relationship between the gut microbiome and brain function. Nutr. Rev. 2018, 76, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Yang, H.; Chen, L. Metabolic regulation on the immune environment of glioma through gut microbiota. Semin. Cancer Biol. 2022, 86, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflammation 2019, 16, 53. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Heng, B.; Guillemin, G.J. The Gut Microbiota, Kynurenine Pathway, and Immune System Interaction in the Development of Brain Cancer. Front. Cell Dev. Biol. 2020, 8, 562812. [Google Scholar] [CrossRef]
- Dono, A.; Nickles, J.; Rodriguez-Armendariz, A.G.; McFarland, B.C.; Ajami, N.J.; Ballester, L.Y.; Wargo, J.A.; Esquenazi, Y. Glioma and the gut-brain axis: Opportunities and future perspectives. Neuro-Oncol. Adv. 2022, 4, vdac054. [Google Scholar] [CrossRef]
- Keane, L.; Cryan, J.F.; Gleeson, J.P. Exploiting the gut microbiome for brain tumour treatment. Trends Mol. Med. 2025, 31, 213–223. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.; Zhang, H.; Zhang, Y.; Ju, H.; Wang, X.; Ren, H.; Zhu, X.; Dong, Y. The immunosuppressive microenvironment and immunotherapy in human glioblastoma. Front. Immunol. 2022, 13, 1003651. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Lauro, C.; Quaglio, D.; Ghirga, F.; Botta, B.; Trettel, F.; Limatola, C. Neuro-Signals from Gut Microbiota: Perspectives for Brain Glioma. Cancers 2021, 13, 2810. [Google Scholar] [CrossRef]
- Naghavian, R.; Faigle, W.; Oldrati, P.; Wang, J.; Toussaint, N.C.; Qiu, Y.; Medici, G.; Wacker, M.; Freudenmann, L.K.; Bonté, P.E.; et al. Microbial peptides activate tumour-infiltrating lymphocytes in glioblastoma. Nature 2023, 617, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, Z.; Peng, M.; Zhang, L.; Wang, C.; Luo, F.; Zeng, M.; Sun, K.; Fang, Z.; Luo, Y.; et al. Multi-omics analysis reveals the interplay between intratumoral bacteria and glioma. mSystems 2025, 10, e0045724. [Google Scholar] [CrossRef]
- Rosito, M.; Maqbool, J.; Reccagni, A.; Giampaoli, O.; Sciubba, F.; Antonangeli, F.; Scavizzi, F.; Raspa, M.; Cordella, F.; Tondo, L.; et al. Antibiotics treatment promotes vasculogenesis in the brain of glioma-bearing mice. Cell Death Dis. 2024, 15, 210. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Antonangeli, F.; Marrocco, F.; Porzia, A.; Lauro, C.; Santoni, A.; Limatola, C. Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. Eur. J. Immunol. 2020, 50, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Dono, A.; Patrizz, A.; McCormack, R.M.; Putluri, N.; Ganesh, B.P.; Kaur, B.; McCullough, L.D.; Ballester, L.Y.; Esquenazi, Y. Glioma induced alterations in fecal short-chain fatty acids and neurotransmitters. CNS Oncol. 2020, 9, CNS57. [Google Scholar] [CrossRef]
- Patrizz, A.; Dono, A.; Zorofchian, S.; Hines, G.; Takayasu, T.; Husein, N.; Otani, Y.; Arevalo, O.; Choi, H.A.; Savarraj, J.; et al. Glioma and temozolomide induced alterations in gut microbiome. Sci. Rep. 2020, 10, 21002. [Google Scholar] [CrossRef]
- Meléndez-Vázquez, N.M.; Nguyen, T.T.; Fan, X.; López-Rivas, A.R.; Fueyo, J.; Gomez-Manzano, C.; Godoy-Vitorino, F. Gut microbiota composition is associated with the efficacy of Delta-24-RGDOX in malignant gliomas. Mol. Ther. Oncol. 2024, 32, 200787. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Fan, H.; Han, M.; Xie, J.; Du, J.; Peng, F. Bifidobacterium lactis combined with Lactobacillus plantarum inhibit glioma growth in mice through modulating PI3K/AKT pathway and gut microbiota. Front. Microbiol. 2022, 13, 986837. [Google Scholar] [CrossRef]
- Jiang, H.; Zeng, W.; Zhang, X.; Pei, Y.; Zhang, H.; Li, Y. The role of gut microbiota in patients with benign and malignant brain tumors: A pilot study. Bioengineered 2022, 13, 7847–7859. [Google Scholar] [CrossRef]
- Wen, Y.; Feng, L.; Wang, H.; Zhou, H.; Li, Q.; Zhang, W.; Wang, M.; Li, Y.; Luan, X.; Jiang, Z.; et al. Association Between Oral Microbiota and Human Brain Glioma Grade: A Case-Control Study. Front. Microbiol. 2021, 12, 746568. [Google Scholar] [CrossRef]
- Cui, C.; Yang, T.; Wang, S.; Jia, Z.; Zhao, L.; Han, X.; Sun, X.; Zong, J.; Wang, S.; Chen, D. Discussion on the relationship between gut microbiota and glioma through Mendelian randomization test based on the brain gut axis. PLoS ONE 2024, 19, e0304403. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yin, F.; Guo, Z.; Li, R.; Sun, W.; Wang, Y.; Geng, Y.; Sun, C.; Sun, D. Association between gut microbiota and glioblastoma: A Mendelian randomization study. Front. Genet. 2024, 14, 1308263. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Zhang, C.; He, C.; Song, H. Investigating the causal impact of gut microbiota on glioblastoma: A bidirectional Mendelian randomization study. BMC Genom. 2023, 24, 784. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Z.; Ma, A.; Li, Z.; Liu, B.; Ma, Q. Computational methods and challenges in analyzing intratumoral microbiome data. Trends Microbiol. 2023, 31, 707–722. [Google Scholar] [CrossRef]
- Park, J.C.; Im, S.H. Of men in mice: The development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp. Mol. Med. 2020, 52, 1383–1396. [Google Scholar] [CrossRef]
- Zucoloto, A.Z.; Yu, I.L.; McCoy, K.D.; McDonald, B. Generation, maintenance, and monitoring of gnotobiotic mice. STAR Protoc. 2021, 2, 100536. [Google Scholar] [CrossRef]
- Yang, S.; Tong, L.; Li, X.; Zhang, Y.; Chen, H.; Zhang, W.; Zhang, H.; Chen, Y.; Chen, R. A novel clinically relevant human fecal microbial transplantation model in humanized mice. Microbiol. Spectr. 2024, 12, e0043624. [Google Scholar] [CrossRef]
| Author/Year | Characteristics | Key Biological Factors | Main Findings |
|---|---|---|---|
| Keane et al., 2025 [8] | Review; gut-brain axis in HGG pathology | Tregs, GAMs, SCFAs, butyrate, immune evasion, BBB integrity | The gut microbiome modulates the glioma microenvironment and may modulate anti-tumor immune responses and therapeutic outcomes |
| Li et al., 2025 [13] | Multi-omics; 50 human glioma patients and GL261 mouse model; investigating intratumoral bacteria | Genera in increased abundance: Fusobacterium, Longibaculum, Pasteurella, Intestinimonas, Arthrobacter, Limosilactobacillus; chemokines CXCL1, CXCL2 and CCL2; neuron-related genes expression | Several bacterial taxa exhibit increased intratumoral occurrence in glioma, notably Fusobacterium nucleatum, which promotes progression by increasing expression of pro-inflammatory chemokines altering the tumor transcriptome |
| Rosito et al., 2024 [14] | Experimental; Murine model study on antibiotic-induced dysbiosis in a GL261 mouse model of glioma | Expression of CD34+, reduced SCFAs and increased glycine concentrations, enhanced vasculogenesis | Dysbiosis promotes vasculogenesis and glioma stemness, shifting the tumor microenvironment toward a pro-angiogenic and pro-tumorigenic phenotype |
| Meléndez-Vázquez et al., 2024 [18] | Original Article; research on gut microbiota and the efficacy of Delta-24-RGDOX oncolytic virotherapy in mice | Prolonged survival in subjects with increased abundance of phyla Verrucomicrobia, Actinobacteria, and genera Akkermansia, Bifidobacterium | Gut microbiota composition influences virotherapy efficacy; specific taxa correlate with enhanced anti-tumor immune responses |
| Cui et al., 2024 [22] | Mendelian Randomization (MR) study exploring causal links between gut microbiota and glioma risk | Specific microbial taxa; neuroinflammation; modulation of CTLA-4, PD-1/PD-L1 | Specific microbial shifts potentially have a causal impact on glioma risk via the gut-brain axis |
| Wang et al., 2024 [23] | Mendelian Randomization (MR) study investigating the association between gut microbiota and glioblastoma | Specific microbial taxa; SCFAs | Evidence of causal associations between specific microbial groups and the risk of developing glioblastoma |
| Naghavian et al., 2023 [11] | Experimental study; Human GBM tissues (n = 12) and T-cell clones | TILs, TCCs, HLA-presented bacterial peptides, phyla Firmicutes, Proteobacteria, Bacteroidetes | Glioblastoma infiltrating lymphocytes recognize microbial peptides presented via HLA-II molecules, suggesting that microbial antigenic mimicry may modulate anti-tumor immunity to favor tumor growth |
| Zeng et al., 2023 [24] | Bidirectional Mendelian Randomization (MR) study on gut microbiota and glioblastoma risk | Specific microbial taxa; both directions causal significance between Prevotella7 genus and glioblastoma | Several microbial taxa show a causal impact on glioblastoma occurrence, reinforcing the role of the gut-brain axis in tumor development |
| Zhang et al., 2022 [9] | Review; immunosuppressive microenvironment in GBM | PD-1/PD-L1, CTLA-4, Th1 cells, Th17 cells, Tregs, GAMs, MDSCs; SCFAs, IL-10, TGF-β, microbial metabolites, tryptophan, neurotransmitters dopamine, serotonin | Gut microbiota modulates anti-tumor immune responses by regulating the GBM-induced immunosuppressive microenvironment and influencing tumor angiogenesis |
| Wang et al., 2022 [19] | Experimental study; original research on the effects of B. lactis and L. plantarum probiotics in glioma-bearing mice | Intestinal barrier integrity, tight junction proteins; the PI3K/AKT pathway; expression of Ki-67 | Probiotic combination inhibits glioma growth by suppressing the PI3K/AKT signaling pathway and reinforcing the tightness of the intestinal barrier |
| Jiang et al., 2022 [20] | Human cohort study; original research comparing gut microbiota in patients with benign (n = 32) and malignant (n = 27) brain tumors | Specific bacterial abundance differences; metabolism pathways alternations: D-glutamine and D-glutamate | Reduced microbial diversity and malignancy-specific taxa in brain tumor patients suggest that gut dysbiosis-linked disruptions may contribute to tumor pathogenesis and progression |
| D’Alessandro et al., 2021 [10] | Review; neuro-signals from the gut microbiota and their role in glioma progression | Dopamine, serotonin, norepinephrine, GABA, glutamate; tumor-driven dysbiosis, reduced F:B ratio, increased Verrucomicrobia phylum | Microbial neuro-active signals may influence tumor progression, while the presence of glioma leads to glioma-induced dysbiosis |
| Wen et al., 2021 [21] | Human case-control study; original research investigating the link between oral microbiota and glioma grade; glioma patients (n = 70) and controls (n = 54) | Occurrence differences in phylum Firmicutes; genera Capnocytophaga, Leptotrichia, Porphyromonas, Haemophilus, Leptotrichia, Capnocytophaga, Bergeyella; species TM7x. LGG, HGG, IDH1 mutation | Oral microbial diversity and specific taxa composition correlate with the pathological grade of glioma |
| Nejman et al., 2020 [12] | Multi-omics; analysis of the intratumor microbiome in 1526 tumors, including brain tumors | Tumour-associated immune cells, intratumoral phyla Proteobacteria, Firmicutes | Diverse neoplasms, including brain tumors, possess distinct, predominantly intracellular microbiome, suggesting a functional interaction between microbial components and immune responses |
| D’Alessandro et al., 2020 [15] | Experimental; short communication; Murine model study investigating gut microbiota and innate immunity in antibiotic-treated GL261 glioma-bearing mice | Families in increased abundance: Alcaligenaceae, Burkholderiaceae and decreased abundance: Prevotellaceae, Rikenellaceae, Helicobacteraceae; reduction in the mature CD27+/CD11b+ NK cells; microglia | Antibiotic-induced dysbiosis impairs NK cell recruitment and shifts microglia toward a pro-tumor phenotype |
| Dono et al., 2020 [16] | Preliminary communication; cross-species project using GL261 mice and human glioma samples to investigate alternations in fecal metabolites and microbiome | Fecal microbial metabolites, SCFAs, neurotransmitters; in glioma-bearing mice decrease in phyla: Bacteroidetes, Firmicutes and increased in phylum Verrucomicrobia | Glioma development alters microbial metabolite profiles, affecting tumor behavior and immune responses in both animal and human models |
| Patrizz et al., 2020 [17] | Longitudinal study; cross-species research on alternations in gut microbiome in mice (GL261) and human glioma patients (n = 12) | In glioma patients and mice: an increase in the phylum Verrucomicrobia, genus Akkermansia, reduced F/B ratio; IDH1 mutation | Glioma development induces gut dysbiosis, exhibiting different mircobial shift signatures between IDH-wildtype and IDH-mutant subtypes |
| Taxonomy | Change in Numerosity | Related Effect | Research Methods and Source |
|---|---|---|---|
| Firmicutes | ↓ | Tumour growth | Murine model (mainly) and cross-species projects, stool examination [10] |
| Bacteroidetes | ↑ | ||
| Verrucomicrobia | ↑ | ||
| Akkermansia muciniphila | ↑ | ||
| Fusobacterium, Fusobacterium nucleatum | ↑ ↑ | Enhanced tumour progression, increased expression of proinflammatory chemokines CXCL1, CXCL2 and CCL2 | 16S rRNA sequencing combined with transcriptomics, metabolomics, IHC, multicolour immunofluorescence, and FISH; animal models [13] |
| Longibaculum | ↑ | ||
| Pasteurella | ↑ | ||
| Intestinimonas | ↑ | ||
| Arthrobacter | ↑ | ||
| Limosilactobacillus | ↑ | ||
| Alcaligenaceae | ↑ | Impaired maturation of cytotoxic natural killer (NK) cells infiltrating brain tissue, reduced mature CD27+/CD11b+ NK cell subset | Murine model, exposition to antibiotics [17] |
| Burkholderiaceae | ↑ | ||
| Prevotellaceae | ↓ | ||
| Rikenellaceae | ↓ | ||
| Helicobacteraceae | ↓ | ||
| Lachnospiraceae | ↓ | Mutation in IDH1-mutant gliomas, typically corresponding to LGG, rare in IDH1–wild-type gliomas, uncommon in grade IV tumours | Oral microbiota examination (saliva samples 16S rRNA sequencing analysis), stratified by malignancy grade, low-grade gliomas (LGG, WHO grades I–II) and high-grade gliomas (HGG, WHO grades III–IV), presence of IDH1 mutation [21] |
| Enterobacteriaceae | ↑ | ||
| Bacteroides | ↑ | ||
| Prevotella | ↑ | ||
| Capnocytophaga | ↓ | Higher glioma grade | |
| Leptotrichia | ↓ | ||
| Porphyromonas | ↓ | ||
| Haemophilus | ↓ | ||
| Capnocytophaga | ↓ | ||
| TM7x | ↓ | ||
| Bergeyella | ↑ | Presence of isocitrate dehydrogenase 1 (IDH1) | |
| Capnocytophaga | ↑ | ||
| Firmicutes | ↓ | ||
| Adlercreutzia | ↑ | Increased glioma risk | Human Mendelian randomization studies, 16S rRNA gene sequencing and host genotyping [22,23,24] |
| Prevotella | ↑ | ||
| Catenibacterium | ↑ | ||
| Coprobacter | ↓ | ||
| Olsenella | ↓ | ||
| Peptostreptococcaceae | ↓ | ||
| Verrucomicrobia | ↑ | ||
| Euryarchaeota | ↑ | ||
| Anaerostipes | ↓ | ||
| Faecalibacterium | ↓ | ||
| Lachnospiraceae UCG004 | ↓ | ||
| Phascolarctobacterium | ↓ | ||
| Streptococcus | ↓ | ||
| Ruminococcaceae | ↓ |
| Molecule | Change in Concentration | Biological Role | Impact on Glioma Progression |
|---|---|---|---|
| Acetate | ↑ | Crossing the BBB, decrease of proinflammatory Th1 and Th17 cells and increase of anti-inflammatory Tregs, neutrophil chemotaxis, enhancement of IL-10 secretion, inhibition of NF-κB signalling, suppression of proinflammatory cytokine production by myeloid cells | ↓ |
| Propionate | ↑ | ↓ | |
| Butyrate | ↑ | ↓ | |
| GABA | ↑ | Reduced proliferation and maintenance of cellular quiescence | ↓ |
| Serotonin | ↑ | Promotion of cell proliferation and migration, induction if differentiation, increase in the release of GDNF | ↑ |
| Dopamine | ↑ | Regulation of cell survival and proliferation, enhancement of the growth of spheroids enriched in cancer stem cells | ↑ |
| Norepinephrine | ↑ | Modulation of proliferation and inhibition of migration and invasion, maintenance of BBB integration | ↓ |
| Acetylcholine | ↑ | Tumour growth, angiogenesis, glioma stemness, tumour proliferation | ↑ |
| Glutamate | ↑ | Cell growth, enhancement of proliferation and migration, promotion of perivascular invasion, overall stimulation of tumour growth and invasiveness | ↑ |
| Glycine | ↑ | Promotion of angiogenesis and cellular stemness, a mediator contributing to glioma progression (in antibiotic-treated mice) | ↑ |
| Tryptophan | ↓ | Gliomas exploit the kynurenine pathway to suppress anti-tumour immune responses via upregulation of IDO1 and TDO, modulated by microbial signals | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Dubiński, P.; Odzimek-Rajska, M.; Podlewski, S.; Brola, W. Emerging Insights into the Role of the Microbiome in Brain Gliomas: A Systematic Review of Recent Evidence. Int. J. Mol. Sci. 2026, 27, 444. https://doi.org/10.3390/ijms27010444
Dubiński P, Odzimek-Rajska M, Podlewski S, Brola W. Emerging Insights into the Role of the Microbiome in Brain Gliomas: A Systematic Review of Recent Evidence. International Journal of Molecular Sciences. 2026; 27(1):444. https://doi.org/10.3390/ijms27010444
Chicago/Turabian StyleDubiński, Piotr, Martyna Odzimek-Rajska, Sebastian Podlewski, and Waldemar Brola. 2026. "Emerging Insights into the Role of the Microbiome in Brain Gliomas: A Systematic Review of Recent Evidence" International Journal of Molecular Sciences 27, no. 1: 444. https://doi.org/10.3390/ijms27010444
APA StyleDubiński, P., Odzimek-Rajska, M., Podlewski, S., & Brola, W. (2026). Emerging Insights into the Role of the Microbiome in Brain Gliomas: A Systematic Review of Recent Evidence. International Journal of Molecular Sciences, 27(1), 444. https://doi.org/10.3390/ijms27010444






