Comprehensive Multimodal and Multiscale Analysis of Alzheimer’s Disease in 5xFAD Mice: Optical Spectroscopies, TEM, Neuropathological, and Behavioral Investigations
Abstract
1. Introduction
2. Results
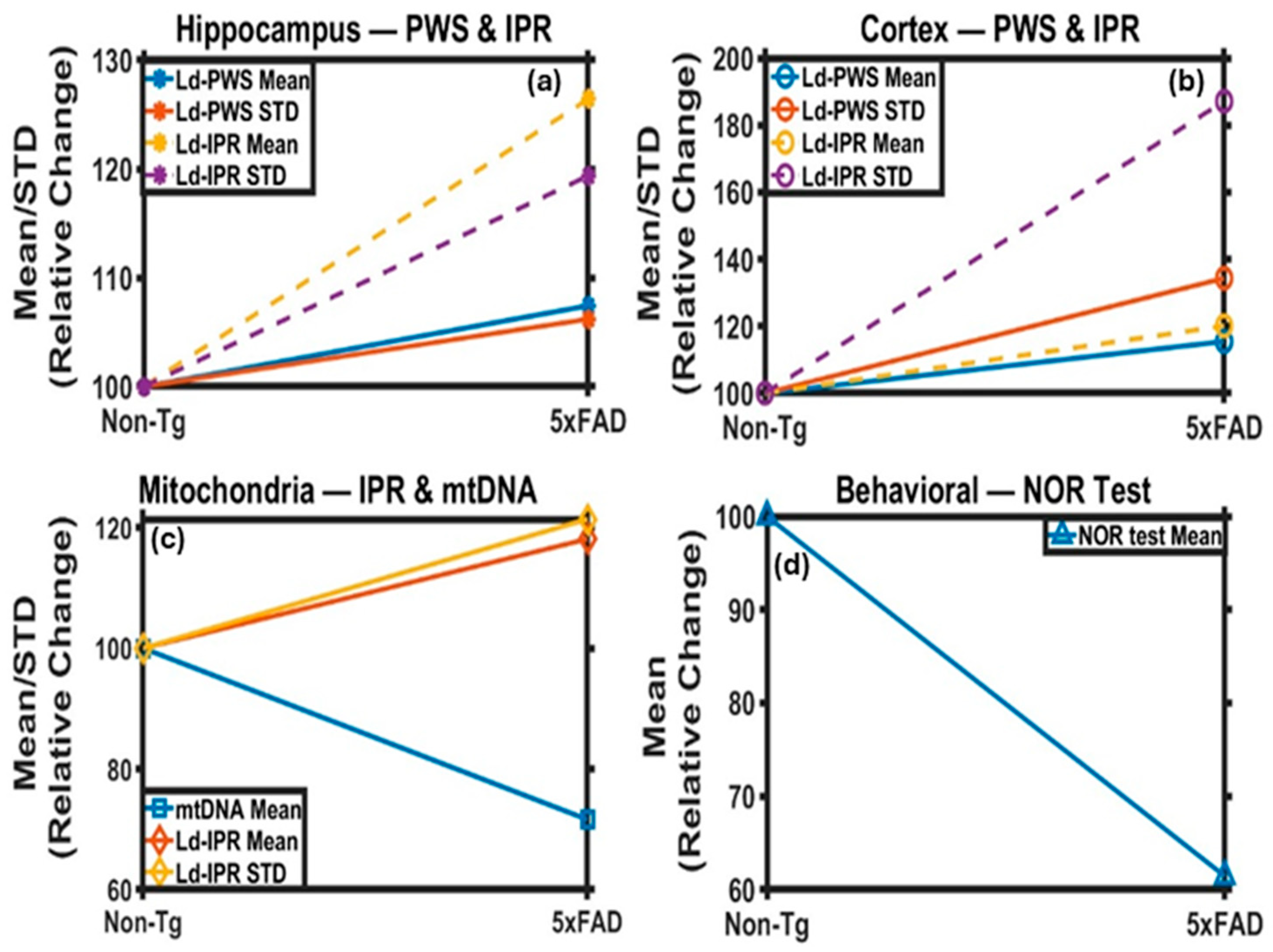
2.1. PWS Analysis of Mice Brain Tissues
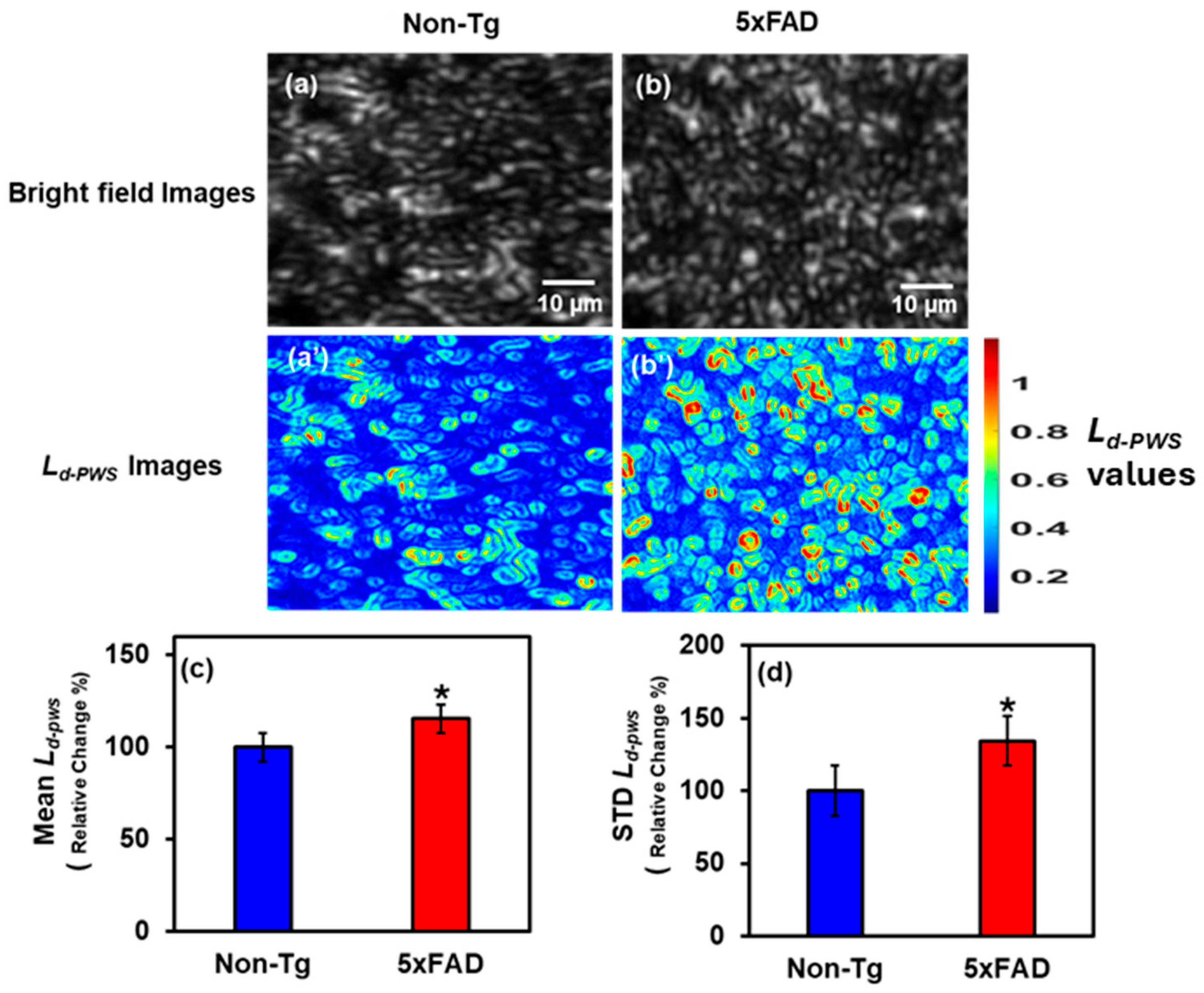
2.1.1. PWS Analysis of Cortical Tissues
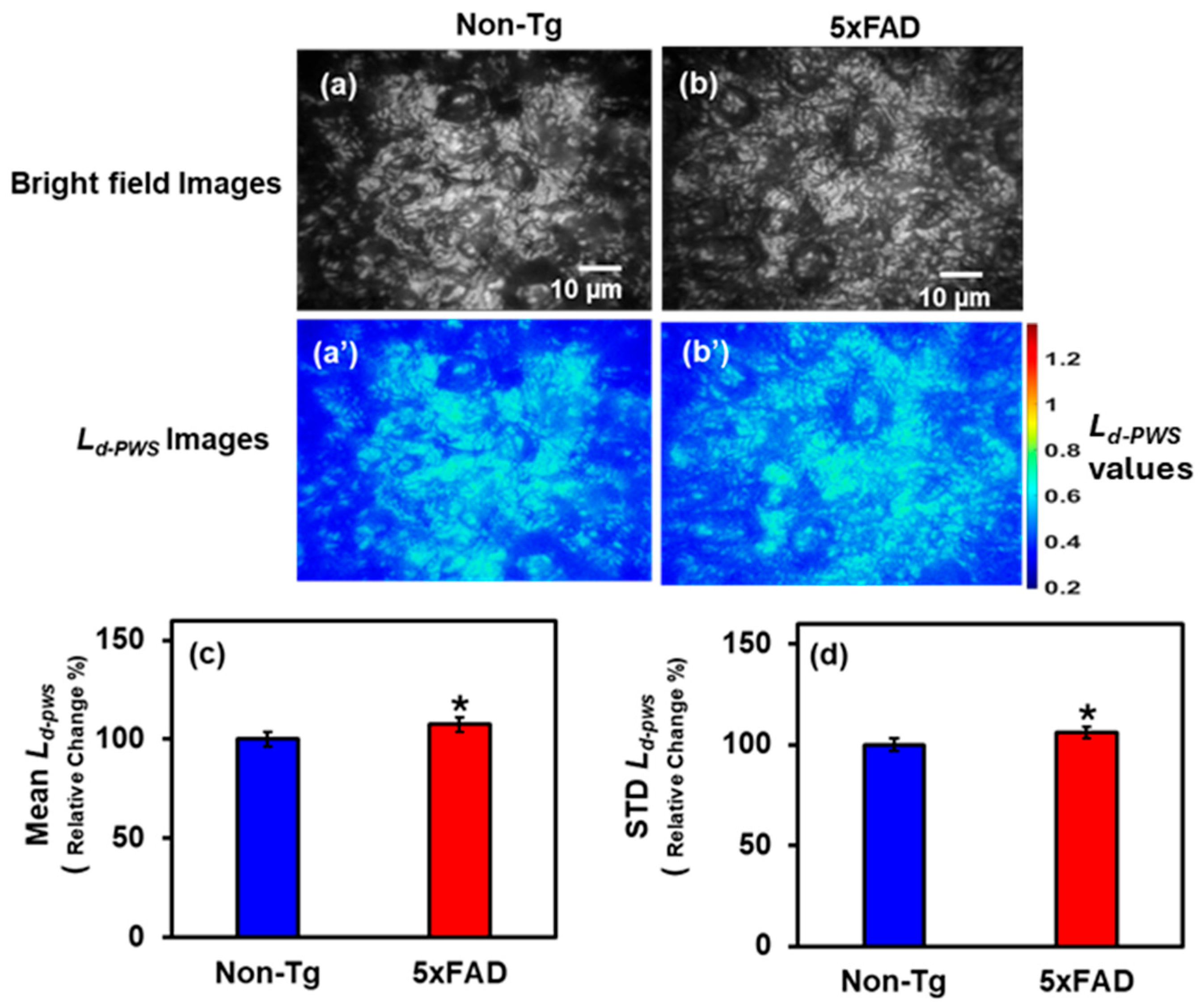
2.1.2. PWS Analysis of Hippocampus Tissues
2.2. IPR Analysis of Mice Brain Tissues
2.2.1. IPR Analysis of Cortex Region
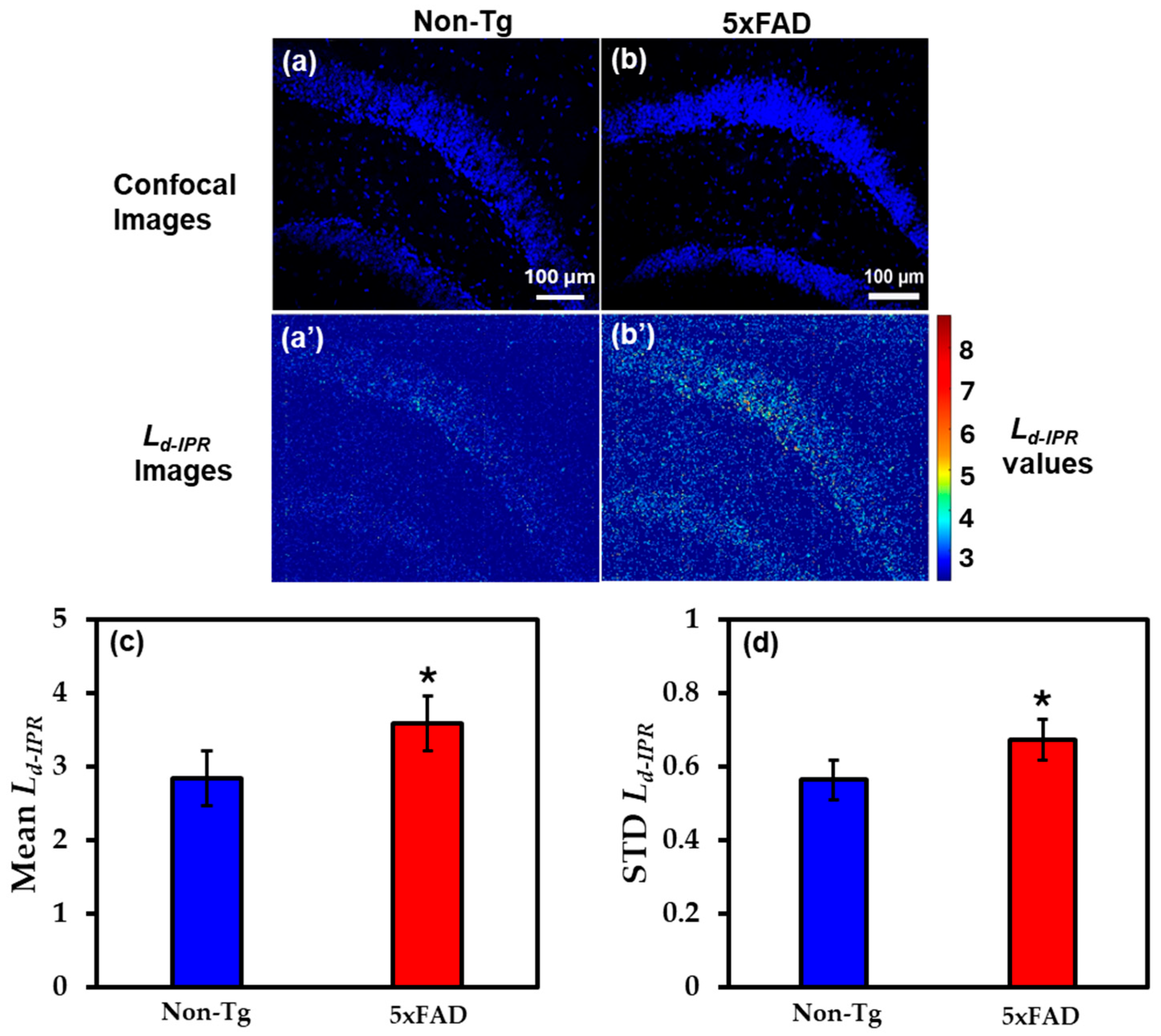
2.2.2. IPR Analysis of Hippocampus Region
2.3. Changes in Mitochondria Structure in 5xFAD Mice: TEM Study
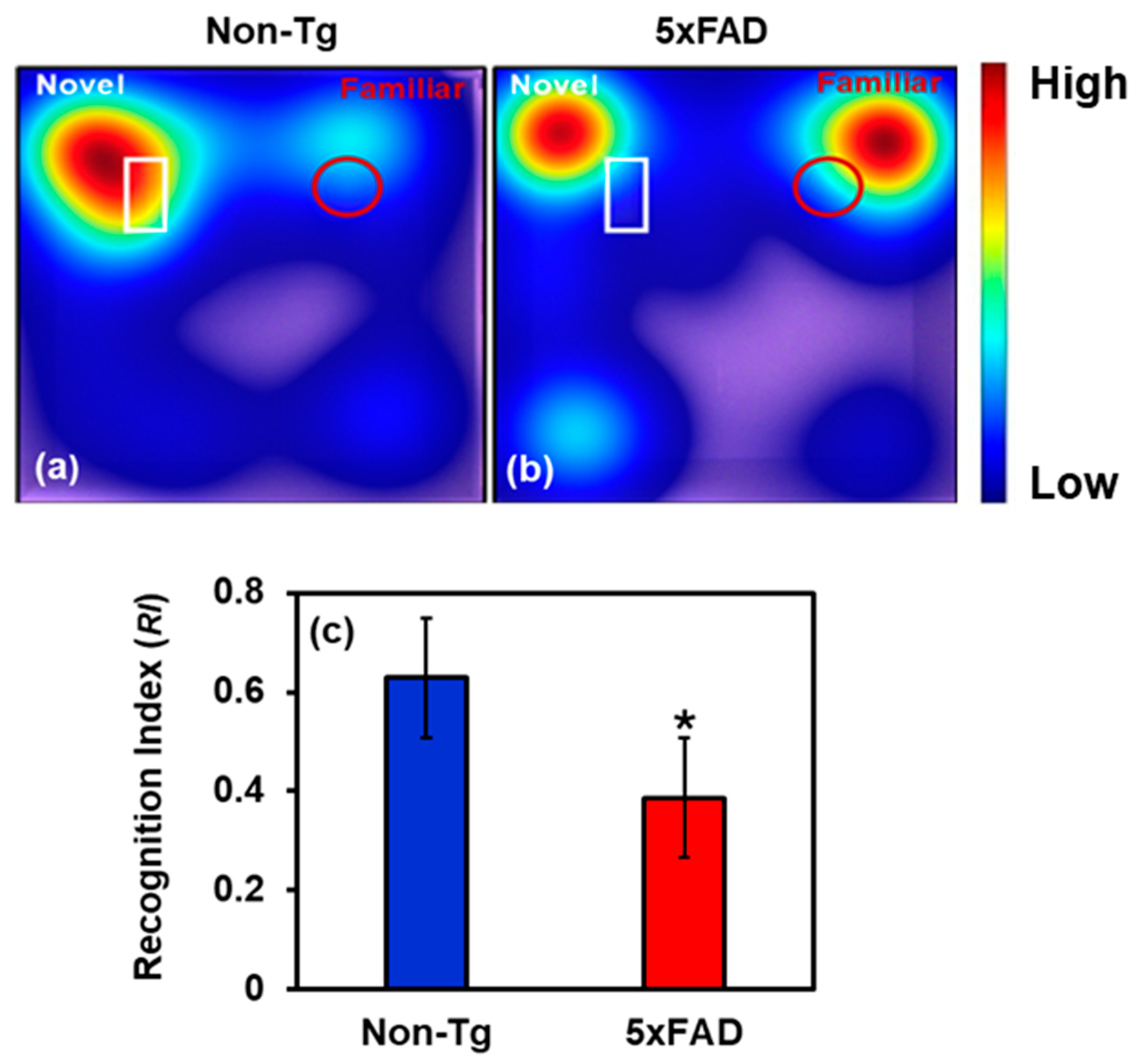
2.4. Behavioral Study
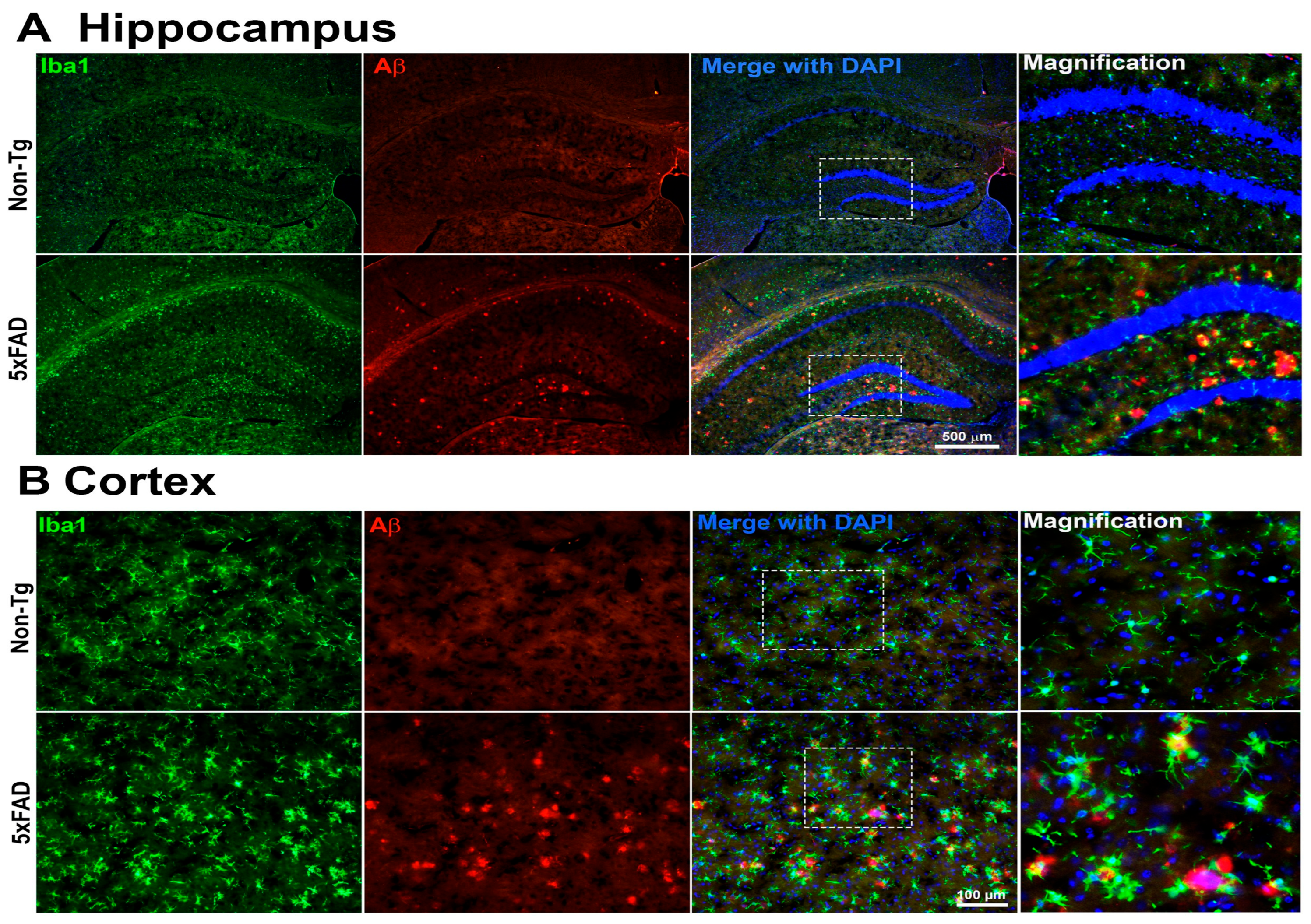
Microglial Activation and Aβ Accumulation in the Brain of 5xFAD Mice
2.5. Mitochondrial DNA Analysis: Relative mtDNA
3. Discussion
4. Materials and Methods
4.1. Partial Wave Spectroscopy Experiment
4.1.1. Optical Setup
4.1.2. Measurement of Structural Disorder Strength
4.1.3. Sample Preparation for PWS Experiment
4.2. Inverse Participation Ratio Quantification Using Confocal Microscopy and Transmission Electron Microscopy
4.2.1. Confocal Imaging
4.2.2. Measurement of IPR
4.2.3. Sample Preparation for IPR Experiment
4.2.4. TEM Imaging of Mitochondria
4.2.5. IPR Quantification Using TEM Images
4.3. Novel Object Recognition
4.4. Immunofluorescence Staining
4.5. Quantification for Mitochondrial DNA Copy Number
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| PWS | Partial Wave Spectroscopy |
| IPR | Inverse Participation Ratio |
| Aβ | Amyloid beta |
| TEM | Transmission Electron Microscope |
| Non-Tg | Non-transgenic |
| mtDNA | Mitochondrial DNA |
| NOR | Novel Object Recognition |
| RI | Refractive Index |
References
- Bush, A.I. The Metallobiology of Alzheimer’s Disease. Trends Neurosci. 2003, 26, 207–214. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2025 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2025, 21, e70235. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H. Alzheimer’s Disease and the β-Amyloid Peptide. J. Alzheimers Dis. 2010, 19, 311. [Google Scholar] [CrossRef] [PubMed]
- Joachim, C.L.; Mori, H.; Selkoe, D.J. Amyloid β-Protein Deposition in Tissues Other than Brain in Alzheimer’s Disease. Nature 1989, 341, 226–230. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s Disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 5 December 2025).
- Li, J.; Li, J. Trends, Inequalities, and Cross-Location Similarities in Global Dementia Burden and Attributable Risk Factors across 204 Countries and Territories: A Systematic Analysis for the Global Burden of Disease Study 2021. Int. J. Surg. 2025, 111, 5298–5310. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of A Beta-Deposition in the Human Brain and Its Relevance for the Development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Marcuello, C.; Lim, K.; Nisini, G.; Pokrovsky, V.S.; Conde, J.; Ruggeri, F.S. Nanoscale Analysis beyond Imaging by Atomic Force Microscopy: Molecular Perspectives on Oncology and Neurodegeneration. Small Sci. 2025, 5, 2500351. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, W.; Wang, L.; Li, J.; Huang, D.; Cheng, W.; Tian, J.; Luan, P. Advance and Prospect of Positron Emission Tomography in Alzheimer’s Disease Research. Mol. Psychiatry 2025, 30, 4899–4909. [Google Scholar] [CrossRef]
- Pradhan, P.; Damania, D.; Joshi, H.M.; Turzhitsky, V.; Subramanian, H.; Roy, H.K.; Taflove, A.; Dravid, V.P.; Backman, V. Quantification of Nanoscale Density Fluctuations by Electron Microscopy: Probing Cellular Alterations in Early Carcinogenesis. Phys. Biol. 2011, 8, 026012. [Google Scholar] [CrossRef]
- Thompson, P.M.; Hayashi, K.M.; de Zubicaray, G.; Janke, A.L.; Rose, S.E.; Semple, J.; Herman, D.; Hong, M.S.; Dittmer, S.S.; Doddrell, D.M.; et al. Dynamics of Gray Matter Loss in Alzheimer’s Disease. J. Neurosci. 2003, 23, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Ribarič, S. Detecting Early Cognitive Decline in Alzheimer’s Disease with Brain Synaptic Structural and Functional Evaluation. Biomedicines 2023, 11, 355. [Google Scholar] [CrossRef]
- Apachigawo, I.; Solanki, D.; Tate, R.; Singh, H.; Khan, M.M.; Pradhan, P. Fractal Dimension Analyses to Detect Alzheimer’s and Parkinson’s Diseases Using Their Thin Brain Tissue Samples via Transmission Optical Microscopy. Biophysica 2023, 3, 569–581. [Google Scholar] [CrossRef]
- Drayer, B.P.; Heyman, A.; Wilkinson, W.; Barrett, L.; Weinberg, T. Early-Onset Alzheimer’s Disease: An Analysis of CT Findings. Ann. Neurol. 1985, 17, 407–410. [Google Scholar] [CrossRef]
- Popp, A.K.; Valentine, M.T.; Kaplan, P.D.; Weitz, D.A. Microscopic Origin of Light Scattering in Tissue. Appl. Opt. 2003, 42, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Boustany, N.N.; Thakor, N.V. Light Scatter Spectroscopy and Imaging of Cellular and Subcellular Events. In Biomedical Photonics: Handbook; CRC Press: Boca Raton, FL, USA, 2003; pp. 437–460. [Google Scholar]
- Drezek, R.; Dunn, A.; Richards-Kortum, R. Light Scattering from Cells: Finite-Difference Time-Domain Simulations and Goniometric Measurements. Appl. Opt. 1999, 38, 3651–3661. [Google Scholar] [CrossRef]
- Adhikari, P.; Alharthi, F.; Pradhan, P. Partial Wave Spectroscopy Detection of Cancer Stages Using Tissue Microarrays (TMA) Samples. In Proceedings of the Frontiers in Optics + Laser Science APS/DLS (2019), Washington, DC, USA, 15–19 September 2019; Optica Publishing Group: Washington, DC, USA, 2019; p. JW4A.89. [Google Scholar]
- Adhikari, P.; Hasan, M.; Sridhar, V.; Roy, D.; Pradhan, P. Studying Nanoscale Structural Alterations in Cancer Cells to Evaluate Ovarian Cancer Drug Treatment, Using Transmission Electron Microscopy Imaging. Phys. Biol. 2020, 17, 036005. [Google Scholar] [CrossRef]
- Alharthi, F.; Apachigawo, I.; Solanki, D.; Khan, S.; Singh, H.; Khan, M.M.; Pradhan, P. Dual Photonics Probing of Nano- to Submicron-Scale Structural Alterations in Human Brain Tissues/Cells and Chromatin/DNA with the Progression of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 12211. [Google Scholar] [CrossRef]
- Liu, P.Y.; Chin, L.K.; Ser, W.; Chen, H.F.; Hsieh, C.-M.; Lee, C.-H.; Sung, K.-B.; Ayi, T.C.; Yap, P.H.; Liedberg, B.; et al. Cell Refractive Index for Cell Biology and Disease Diagnosis: Past, Present and Future. Lab Chip 2016, 16, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Gladstein, S.; Damania, D.; Almassalha, L.M.; Smith, L.T.; Gupta, V.; Subramanian, H.; Rex, D.K.; Roy, H.K.; Backman, V. Correlating Colorectal Cancer Risk with Field Carcinogenesis Progression Using Partial Wave Spectroscopic Microscopy. Cancer Med. 2018, 7, 2109–2120. [Google Scholar] [CrossRef]
- Backman, V.; Roy, H.K. Light-Scattering Technologies for Field Carcinogenesis Detection: A Modality for Endoscopic Prescreening. Gastroenterology 2011, 140, 35–41.e5. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Pradhan, P.; Liu, Y.; Capoglu, I.R.; Li, X.; Rogers, J.D.; Heifetz, A.; Kunte, D.; Roy, H.K.; Taflove, A.; et al. Optical Methodology for Detecting Histologically Unapparent Nanoscale Consequences of Genetic Alterations in Biological Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 20118–20123. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Wisniewski, T. Alzheimer’s Disease: Experimental Models and Reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- New Genetically Modified Mouse Model Mimics Multiple Aspects of Human Alzheimer’s Disease. Available online: https://www.nia.nih.gov/news/new-genetically-modified-mouse-model-mimics-multiple-aspects-human-alzheimers-disease (accessed on 2 December 2024).
- Hoekstra, J.G.; Hipp, M.J.; Montine, T.J.; Kennedy, S.R. Mitochondrial DNA Mutations Increase in Early Stage Alzheimer’s Disease and Are Inconsistent with Oxidative Damage. Ann. Neurol. 2016, 80, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H. Mitochondrial DNA–Related Mitochondrial Dysfunction in Neurodegenerative Diseases. Arch. Pathol. Lab Med. 2002, 126, 271–280. [Google Scholar] [CrossRef]
- Tsering, W.; Prokop, S. Neuritic Plaques—Gateways to Understanding Alzheimer’s Disease. Mol. Neurobiol. 2024, 61, 2808–2821. [Google Scholar] [CrossRef]
- Gazestani, V.; Kamath, T.; Nadaf, N.M.; Dougalis, A.; Burris, S.J.; Rooney, B.; Junkkari, A.; Vanderburg, C.; Pelkonen, A.; Gomez-Budia, M.; et al. Early Alzheimer’s Disease Pathology in Human Cortex Involves Transient Cell States. Cell 2023, 186, 4438–4453.e23. [Google Scholar] [CrossRef]
- Kobro-Flatmoen, A.; Lagartos-Donate, M.J.; Aman, Y.; Edison, P.; Witter, M.P.; Fang, E.F. Re-Emphasizing Early Alzheimer’s Disease Pathology Starting in Select Entorhinal Neurons, with a Special Focus on Mitophagy. Ageing Res. Rev. 2021, 67, 101307. [Google Scholar] [CrossRef]
- Thadathil, N.; Delotterie, D.F.; Xiao, J.; Hori, R.; McDonald, M.P.; Khan, M.M. DNA Double-Strand Break Accumulation in Alzheimer’s Disease: Evidence from Experimental Models and Postmortem Human Brains. Mol. Neurobiol. 2021, 58, 118–131. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Huang, M.; Gunewardena, S.; Haeri, M.; Swerdlow, R.H.; Wang, N. Landscape of Double-Stranded DNA Breaks in Postmortem Brains from Alzheimer’s Disease and Non-Demented Individuals. J. Alzheimers Dis. 2023, 94, 519–535. [Google Scholar] [CrossRef]
- Dileep, V.; Boix, C.A.; Mathys, H.; Marco, A.; Welch, G.M.; Meharena, H.S.; Loon, A.; Jeloka, R.; Peng, Z.; Bennett, D.A.; et al. Neuronal DNA Double-Strand Breaks Lead to Genome Structural Variations and 3D Genome Disruption in Neurodegeneration. Cell 2023, 186, 4404–4421.e20. [Google Scholar] [CrossRef] [PubMed]
- Mengel-From, J.; Thinggaard, M.; Dalgård, C.; Kyvik, K.O.; Christensen, K.; Christiansen, L. Mitochondrial DNA Copy Number in Peripheral Blood Cells Declines with Age and Is Associated with General Health among Elderly. Hum. Genet. 2014, 133, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Filograna, R.; Mennuni, M.; Alsina, D.; Larsson, N.-G. Mitochondrial DNA Copy Number in Human Disease: The More the Better? FEBS Lett. 2021, 595, 976–1002. [Google Scholar] [CrossRef] [PubMed]
- Cerantonio, A.; Citrigno, L.; Greco, B.M.; De Benedittis, S.; Passarino, G.; Maletta, R.; Qualtieri, A.; Montesanto, A.; Spadafora, P.; Cavalcanti, F. The Role of Mitochondrial Copy Number in Neurodegenerative Diseases: Present Insights and Future Directions. Int. J. Mol. Sci. 2024, 25, 6062. [Google Scholar] [CrossRef]
- Castellani, C.A.; Longchamps, R.J.; Sun, J.; Guallar, E.; Arking, D.E. Thinking Outside the Nucleus: Mitochondrial DNA Copy Number in Health and Disease. Mitochondrion 2020, 53, 214–223. [Google Scholar] [CrossRef]
- D’Alessandro, M.C.B.; Kanaan, S.; Geller, M.; Praticò, D.; Daher, J.P.L. Mitochondrial Dysfunction in Alzheimer’s Disease. Ageing Res. Rev. 2025, 107, 102713. [Google Scholar] [CrossRef]
- Perez Ortiz, J.M.; Swerdlow, R.H. Mitochondrial Dysfunction in Alzheimer’s Disease: Role in Pathogenesis and Novel Therapeutic Opportunities. Br. J. Pharmacol. 2019, 176, 3489–3507. [Google Scholar] [CrossRef]
- Harerimana, N.V.; Paliwali, D.; Romero-Molina, C.; Bennett, D.A.; Pa, J.; Goate, A.; Swerdlow, R.H.; Andrews, S.J. The Role of Mitochondrial Genome Abundance in Alzheimer’s Disease. Alzheimers Dement. 2023, 19, 2069–2083. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wiggins, K.L.; Kurniansyah, N.; Guo, X.; Rodrigue, A.L.; Zhao, W.; Yanek, L.R.; Ratliff, S.M.; Pitsillides, A.; et al. Association of Mitochondrial DNA Copy Number With Brain MRI Markers and Cognitive Function: A Meta-Analysis of Community-Based Cohorts. Neurology 2023, 100, e1930–e1943. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, H.-A.; Han, Y.S.; Jeon, W.K.; Han, J.-S. Recognition Memory Impairments and Amyloid-Beta Deposition of the Retrosplenial Cortex at the Early Stage of 5XFAD Mice. Physiol. Behav. 2020, 222, 112891. [Google Scholar] [CrossRef]
- Pádua, M.S.; Guil-Guerrero, J.L.; Lopes, P.A. Behaviour Hallmarks in Alzheimer’s Disease 5xFAD Mouse Model. Int. J. Mol. Sci. 2024, 25, 6766. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal Beta-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Rajendran, L.; Paolicelli, R.C. Microglia-Mediated Synapse Loss in Alzheimer’s Disease. J. Neurosci. 2018, 38, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of Pro-Inflammatory Cytokines Released from Microglia in Neurodegenerative Diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Romero-Bueno, R.; de la Cruz Ruiz, P.; Artal-Sanz, M.; Askjaer, P.; Dobrzynska, A. Nuclear Organization in Stress and Aging. Cells 2019, 8, 664. [Google Scholar] [CrossRef]
- Gauthier, B.R.; Comaills, V. Nuclear Envelope Integrity in Health and Disease: Consequences on Genome Instability and Inflammation. Int. J. Mol. Sci. 2021, 22, 7281. [Google Scholar] [CrossRef]
- Shukla, P.K.; Delotterie, D.F.; Xiao, J.; Pierre, J.F.; Rao, R.; McDonald, M.P.; Khan, M.M. Alterations in the Gut-Microbial-Inflammasome-Brain Axis in a Mouse Model of Alzheimer’s Disease. Cells 2021, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Shukla, P.; Almabadi, H.; Sahay, P.; Rao, R.; Pradhan, P. Optical Study of Stress Hormone-Induced Nanoscale Structural Alteration in Brain Using Partial Wave Spectroscopic (PWS) Microscopy. J. Biophotonics 2018, 12, e201800002. [Google Scholar] [CrossRef]
- Pradhan, P.; Subramanian, H.; Liu, Y.; Kim, Y.; Roy, H.; Backman, V. Application of Mesoscopic Light Transport Theory to Ultra-Early Detection of Cancer in a Single Biological Cell. In Proceedings of the 2007 APS March Meeting, Denver, CO, USA, 5–9 March 2007; p. B41.013. [Google Scholar]
- Pradhan, P. Phase Statistics of Light Wave Reflected from One-Dimensional Optical Disordered Media and Its Effects on Light Transport Properties. Photonics 2021, 8, 485. [Google Scholar] [CrossRef]
- Pradhan, P.; Liu, Y.; Kim, Y.; Li, X.; Wali, R.K.; Roy, H.K.; Backman, V. Mesoscopic Light Transport Properties of a Single Biological Cell: Early Detection of Cancer. In Proceedings of the 2006 APS March Meeting, Baltimore, MD, USA, 13–17 March 2006; p. Q1.326. [Google Scholar]
- Pradhan, P.; Subramanian, H.; Damania, D.; Roy, H.; Backman, V. Mesoscopic Light Reflection Spectroscopy of Weakly Disordered Dielectric Media: Nanoscopic to Mesoscopic Light Transport Properties of a Single Biological Cell and Ultra-Early Detection of Cancer. In Proceedings of the 2009 APS March Meeting, Pittsburgh, PA, USA, 16–20 March 2009; p. A28.001. [Google Scholar]
- Pradhan, P.; Kumar, N. Localization of Light in Coherently Amplifying Random Media. Phys. Rev. B 1994, 50, 9644–9647. [Google Scholar] [CrossRef]
- Roy, H.K.; Subramanian, H.; Damania, D.; Hensing, T.A.; Rom, W.N.; Pass, H.I.; Ray, D.; Rogers, J.D.; Bogojevic, A.; Shah, M.; et al. Optical Detection of Buccal Epithelial Nanoarchitectural Alterations in Patients Harboring Lung Cancer: Implications for Screening. Cancer Res. 2010, 70, 7748–7754. [Google Scholar] [CrossRef]
- Subramanian, H.; Pradhan, P.; Kunte, D.; Deep, N.; Roy, H.; Backman, V. Single-Cell Partial Wave Spectroscopic Microscopy. In Proceedings of the Biomedical Optics (2008), St. Petersburg, FL, USA, 16–19 March 2008; Optica Publishing Group: Washington, DC, USA, 2008; p. BTuC5. [Google Scholar]
- Pradhan, P.; Damania, D.; Joshi, H.M.; Turzhitsky, V.; Subramanian, H.; Roy, H.K.; Taflove, A.; Dravid, V.P.; Backman, V. Quantification of Nanoscale Density Fluctuations Using Electron Microscopy: Light-Localization Properties of Biological Cells. Appl. Phys. Lett. 2010, 97, 243704. [Google Scholar] [CrossRef]
- Lee, P.A.; Fisher, D.S. Anderson Localization in Two Dimensions. Phys. Rev. Lett. 1981, 47, 882–885. [Google Scholar] [CrossRef]
- Mafi, A. Transverse Anderson Localization of Light: A Tutorial. Adv. Opt. Photon. 2015, 7, 459–515. [Google Scholar] [CrossRef]
- Adhikari, P.; Shukla, P.K.; Rao, R.; Pradhan, P. Quantification of Light Localization Properties to Study the Effect of Probiotic on Chronic Alcoholic Brain Cells via Confocal Imaging. In Proceedings of the Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues XIX, Online Only, USA, 6–11 March 2021; Leary, J.F., Tarnok, A., Georgakoudi, I., Eds.; SPIE: Bellingham, WA, USA, 2021; p. 41. [Google Scholar]
- Singh, H.; Khan, S.; Xiao, J.; Nguyen, N.; Das, A.; Johnson, D.; Fang-Liao, F.; Frautschy, S.A.; McDonald, M.P.; Pourmotabbed, T.; et al. Harnessing cGAS-STING Signaling to Counteract the Genotoxic-Immune Nexus in Tauopathy. bioRxiv 2025. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 126, 55718. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, X. Behavioral and Pathological Characteristics of 5xFAD Female Mice in the Early Stage. Sci. Rep. 2025, 15, 6924. [Google Scholar] [CrossRef]
- Khan, S.; Delotterie, D.F.; Xiao, J.; Thangavel, R.; Hori, R.; Koprich, J.; Alway, S.E.; McDonald, M.P.; Khan, M.M. Crosstalk between DNA Damage and cGAS-STING Immune Pathway Drives Neuroinflammation and Dopaminergic Neurodegeneration in Parkinson’s Disease. Brain Behav. Immun. 2025, 130, 106065. [Google Scholar] [CrossRef]



| Primer | Sequence (5′→3′) | Locus | Species | Product (bp) |
|---|---|---|---|---|
| mtDNA_mF1 | cagaaacaaaccgggccc | NC_005089.1 3322-3339 | Mouse | |
| mtDNA_mR1 | gccggctgcgtattctac | NC_005089.1 3404-3387 | Mouse | 83 (with mtDNA_mF1) |
| nDNA_mF1 | ccagggagagctagtatctagg | NC_000072 122150920-0941 | Mouse | |
| nDNA_mR1 | ctggtcatgggagaaaaggc | NC_000072 122151095-1076 | Mouse | 176 (with nDNA_mF1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Solanki, D.; Apachigawo, I.; Khan, S.; Maity, S.; Alharthi, F.; Nasim, S.; Sweety, F.; Alizadeh Poshtiri, M.; Xiao, J.; Khan, M.M.; et al. Comprehensive Multimodal and Multiscale Analysis of Alzheimer’s Disease in 5xFAD Mice: Optical Spectroscopies, TEM, Neuropathological, and Behavioral Investigations. Int. J. Mol. Sci. 2026, 27, 198. https://doi.org/10.3390/ijms27010198
Solanki D, Apachigawo I, Khan S, Maity S, Alharthi F, Nasim S, Sweety F, Alizadeh Poshtiri M, Xiao J, Khan MM, et al. Comprehensive Multimodal and Multiscale Analysis of Alzheimer’s Disease in 5xFAD Mice: Optical Spectroscopies, TEM, Neuropathological, and Behavioral Investigations. International Journal of Molecular Sciences. 2026; 27(1):198. https://doi.org/10.3390/ijms27010198
Chicago/Turabian StyleSolanki, Dhruvil, Ishmael Apachigawo, Sazzad Khan, Santanu Maity, Fatemah Alharthi, Samia Nasim, Fnu Sweety, Mohammad Alizadeh Poshtiri, Jianfeng Xiao, Mohammad Moshahid Khan, and et al. 2026. "Comprehensive Multimodal and Multiscale Analysis of Alzheimer’s Disease in 5xFAD Mice: Optical Spectroscopies, TEM, Neuropathological, and Behavioral Investigations" International Journal of Molecular Sciences 27, no. 1: 198. https://doi.org/10.3390/ijms27010198
APA StyleSolanki, D., Apachigawo, I., Khan, S., Maity, S., Alharthi, F., Nasim, S., Sweety, F., Alizadeh Poshtiri, M., Xiao, J., Khan, M. M., & Pradhan, P. (2026). Comprehensive Multimodal and Multiscale Analysis of Alzheimer’s Disease in 5xFAD Mice: Optical Spectroscopies, TEM, Neuropathological, and Behavioral Investigations. International Journal of Molecular Sciences, 27(1), 198. https://doi.org/10.3390/ijms27010198






