Plasma Levels of Food-Derived Metabolites as Biomarkers of Parkinson’s Disease
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Caffeine
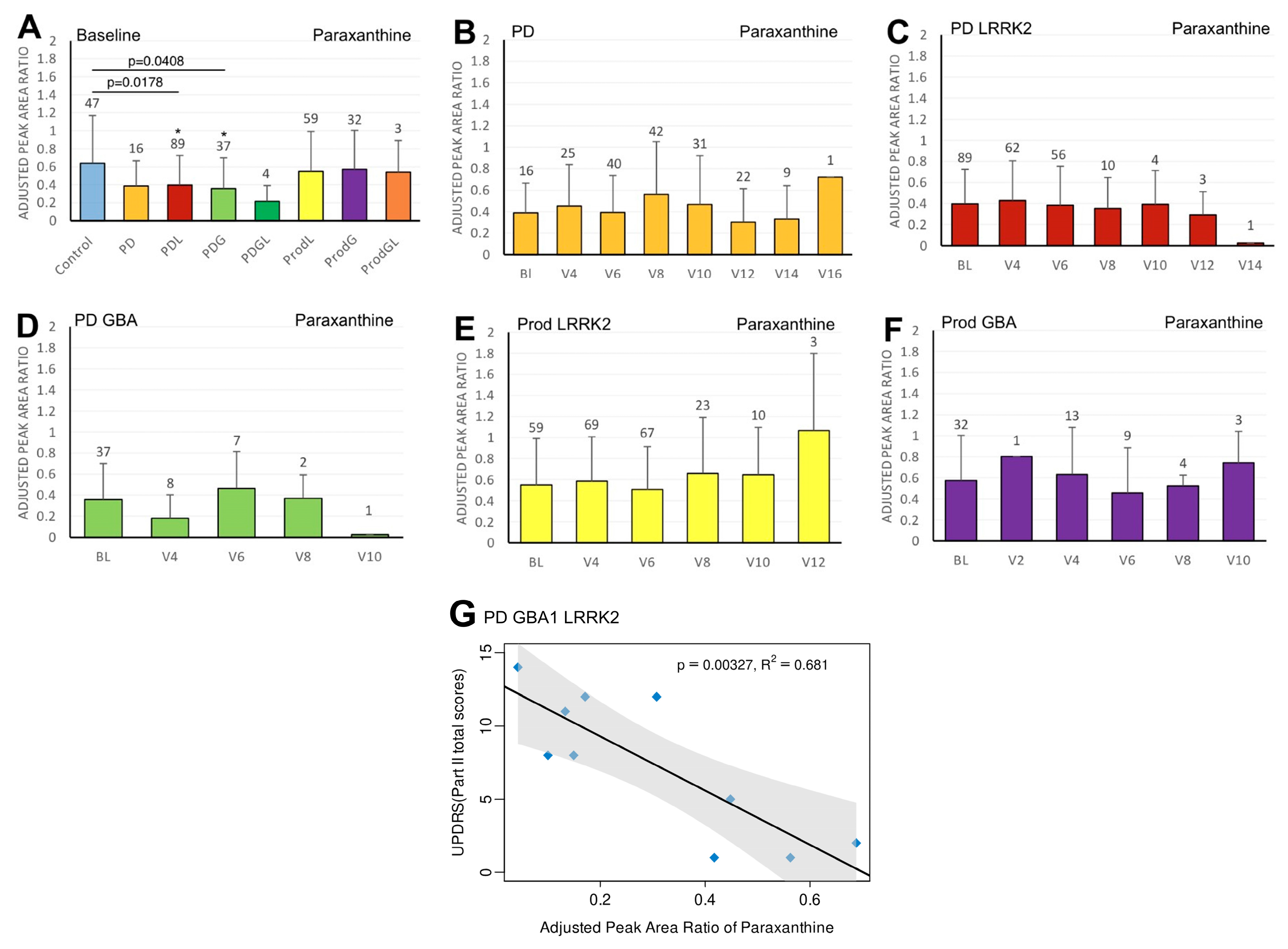
3.2. Paraxanthine
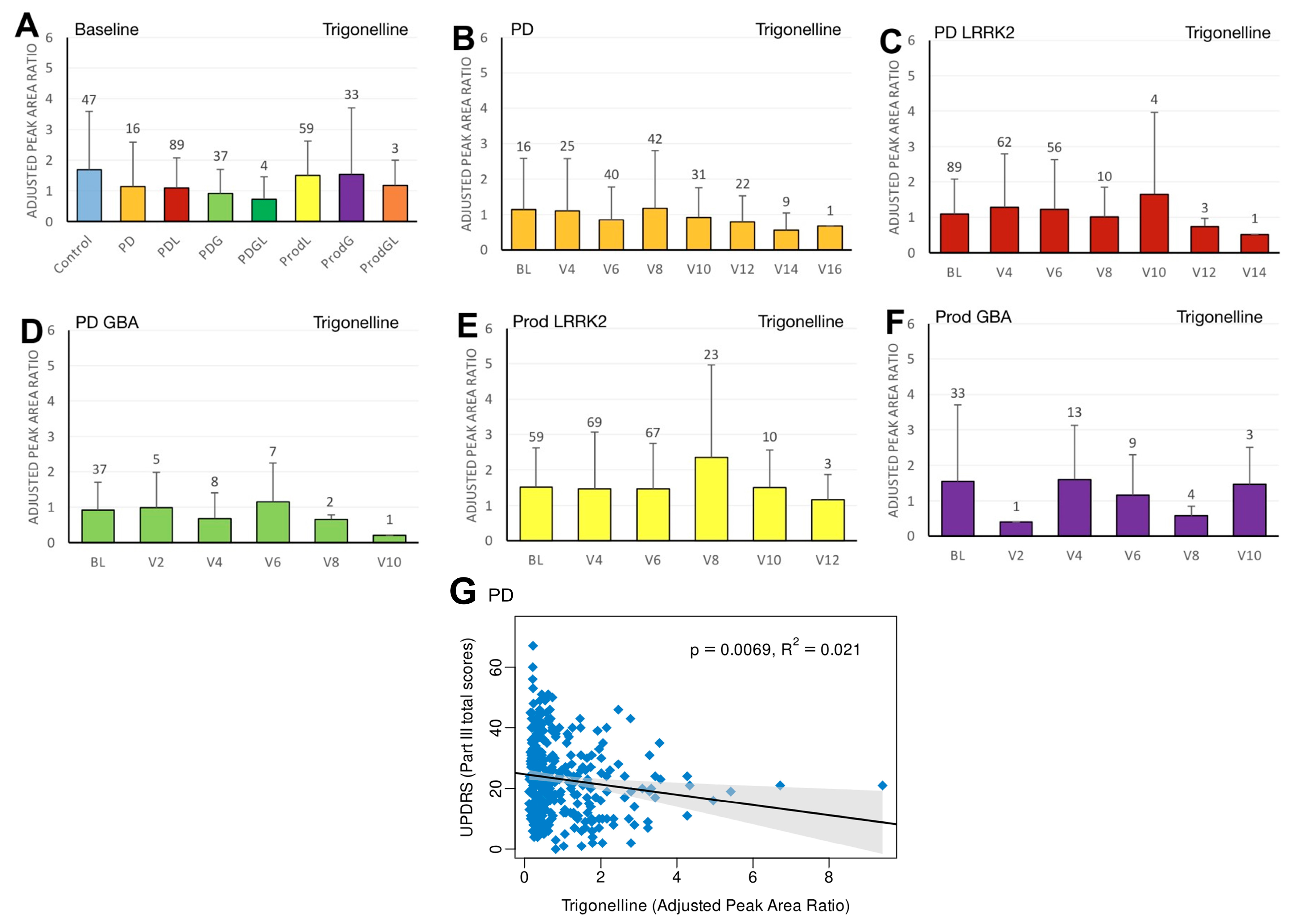
3.3. Trigonelline
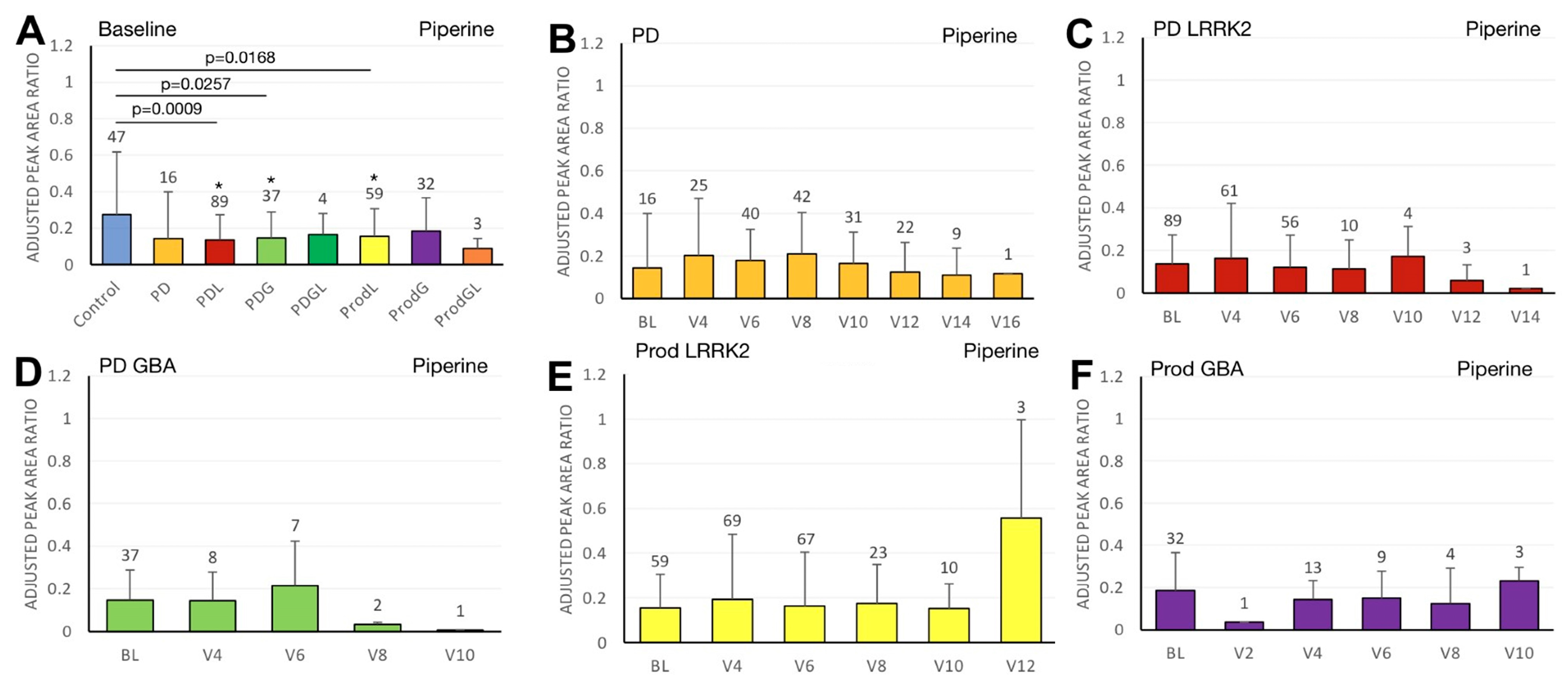
3.4. Piperine
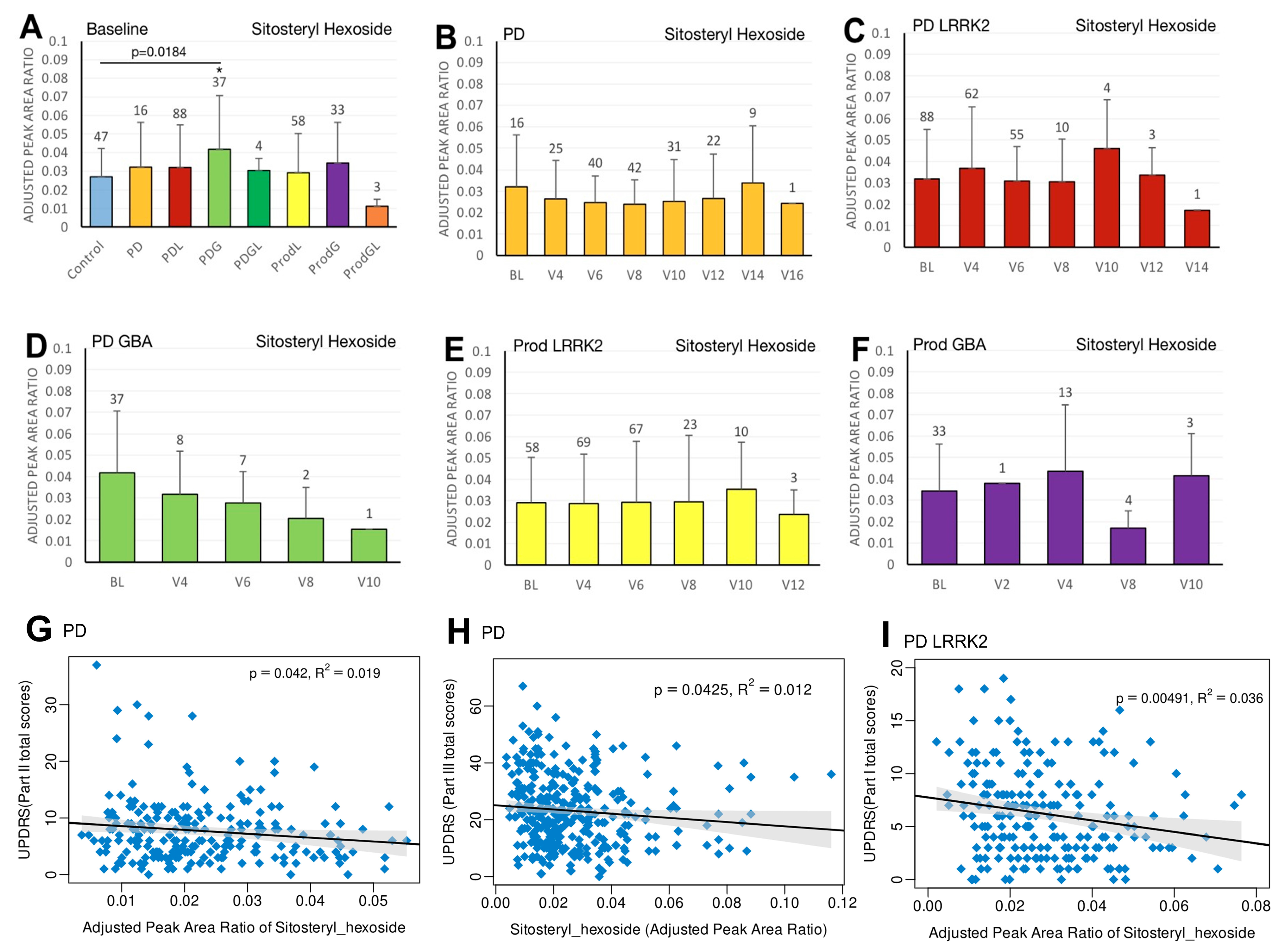
3.5. Sitosteryl Hexoside
3.6. Practical Clinical Recommendations
3.7. Strengths and Future Directions
4. Materials and Methods
4.1. Study Design
4.2. LC/MS Protocol
4.3. Plasma Biomarker Quantification
4.4. LRRK2 and GBA Genotyping
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Rossi, A.; Berger, K.; Chen, H.; Leslie, D.; Mailman, R.B.; Huang, X. Projection of the prevalence of Parkinson’s disease in the coming decades: Revisited. Mov. Disord. 2018, 33, 156–159. [Google Scholar] [CrossRef]
- Yamashita, K.Y.; Bhoopatiraju, S.; Silverglate, B.D.; Grossberg, G.T. Biomarkers in Parkinson’s disease: A state of the art review. Biomark. Neuropsychiatry 2023, 9, 100074. [Google Scholar] [CrossRef]
- Chen, C.C.; Chiu, J.Y.; Tan, A.H.; Toh, T.S.; Lim, S.Y.; Tan, E.K.; Pettersson, S.; Hsu, C.C.; Liou, J.M.; Wu, M.S.; et al. Investigating Plasma Metabolomics and Gut Microbiota Changes Associated with Parkinson Disease: A Focus on Caffeine Metabolism. Neurology 2025, 104, e213592. [Google Scholar] [CrossRef]
- Flores-Torres, M.H.; Peng, X.; Jeanfavre, S.; Clish, C.; Wang, Y.; McCullough, M.L.; Healy, B.; Schwarzschild, M.A.; Bjornevik, K.; Ascherio, A. Plasma Metabolomics Profiles in Prodromal and Clinical Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2025, 40, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lai, Y.; Konijnenberg, H.; Huerta, J.M.; Vinagre-Aragon, A.; Sabin, J.A.; Hansen, J.; Petrova, D.; Sacerdote, C.; Zamora-Ros, R.; et al. Association of Coffee Consumption and Prediagnostic Caffeine Metabolites with Incident Parkinson Disease in a Population-Based Cohort. Neurology 2024, 102, e209201. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouzan, M.; Hamadeh, M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef]
- Mirzaie, M.; Khalili, M.; Kiasalari, Z.; Roghani, M.J.N. Neuroprotective and antiapoptotic potential of trigonelline in a striatal 6-hydroxydopamine rat model of Parkinson’s disease. Neurophysiology 2016, 48, 176–183. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Y.-H.; Liu, H.; Qu, H.-D. Neuroprotective effects of piperine on the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced Parkinson’s disease mouse model. Int. J. Mol. Med. 2015, 36, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tan, M.A.; An, S.S.A. Phytosterols: Potential Metabolic Modulators in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 12255. [Google Scholar] [CrossRef] [PubMed]
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Kachergus, J.; Hulihan, M.; Uitti, R.J.; Calne, D.B.; et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44, 601–607. [Google Scholar] [CrossRef]
- Sidransky, E.; Nalls, M.A.; Aasly, J.O.; Aharon-Peretz, J.; Annesi, G.; Barbosa, E.R.; Bar-Shira, A.; Berg, D.; Bras, J.; Brice, A.; et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 2009, 361, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Crotty, G.F.; Maciuca, R.; Macklin, E.A.; Wang, J.; Montalban, M.; Davis, S.S.; Alkabsh, J.I.; Bakshi, R.; Chen, X.; Ascherio, A.J.N. Association of caffeine and related analytes with resistance to Parkinson disease among LRRK2 mutation carriers: A metabolomic study. Neurology 2020, 95, e3428–e3437. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Tang, B.; Guo, J. Clinical, mechanistic, biomarker, and therapeutic advances in GBA1-associated Parkinson’s disease. Transl. Neurodegener. 2024, 13, 48. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Z.D.; Yi, L.; Tan, B.J.-W.; Tan, E.-K. Interaction between caffeine consumption & genetic susceptibility in Parkinson’s disease: A systematic review. Ageing Res. Rev. 2024, 99, 102381. [Google Scholar] [CrossRef]
- McGhee, D.J.M.; Royle, P.L.; Thompson, P.A.; Wright, D.E.; Zajicek, J.P.; Counsell, C.E. A systematic review of biomarkers for disease progression in Parkinson’s disease. BMC Neurol. 2013, 13, 35. [Google Scholar] [CrossRef]
- Marek, K.; Jennings, D.; Lasch, S.; Siderowf, A.; Tanner, C.; Simuni, T.; Coffey, C.; Kieburtz, K.; Flagg, E.; Chowdhury, S.; et al. The Parkinson Progression Marker Initiative (PPMI). Prog. Neurobiol. 2011, 95, 629–635. [Google Scholar] [CrossRef]
- Guerreiro, S.; Toulorge, D.; Hirsch, E.; Marien, M.; Sokoloff, P.; Michel, P.P. Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol. Pharmacol. 2008, 74, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Vaibhav, K.; Tabassum, R.; Khan, A.; Ishrat, T.; Khan, M.M.; Ahmad, A.; Islam, F.; Safhi, M.M.; Islam, F. Anti-apoptotic and anti-inflammatory effect of Piperine on 6-OHDA induced Parkinson’s rat model. J. Nutr. Biochem. 2013, 24, 680–687. [Google Scholar] [CrossRef]
- Dash, R.; Mitra, S.; Ali, M.C.; Oktaviani, D.F.; Hannan, M.A.; Choi, S.M.; Moon, I.S. Phytosterols: Targeting neuroinflammation in neurodegeneration. Curr. Pharm. Des. 2021, 27, 383–401. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A.; et al. Anti-Alzheimer’s studies on β-sitosterol isolated from Polygonum hydropiper L. Front. Pharmacol. 2017, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wu, F.; Xu, J. Incorporation of β-sitosterol into mitochondrial membrane enhances mitochondrial function by promoting inner mitochondrial membrane fluidity. J. Bioenerg. Biomembr. 2013, 45, 301–305. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. β-Sitosterol alleviates inflammatory response via inhibiting the activation of ERK/p38 and NF-κB pathways in LPS-exposed BV2 Cells. BioMed Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, M.; Saiki, S.; Li, Y.; Kaga, N.; Taka, H.; Hatano, T.; Ishikawa, K.-I.; Oji, Y.; Mori, A.; Okuzumi, A.; et al. Serum caffeine and metabolites are reliable biomarkers of early Parkinson disease. Neurology 2018, 90, e404–e411. [Google Scholar] [CrossRef]
- Walton-Doyle, C.; Menozzi, E.; Schapira, A.H.; Barran, P. Metabolomic Changes in Idiopathic and GBA1 Parkinson’s Disease. medRxiv 2025. [Google Scholar] [CrossRef]
- Postuma, R.B.; Lang, A.E.; Munhoz, R.P.; Charland, K.; Pelletier, A.; Moscovich, M.; Filla, L.; Zanatta, D.; Rios Romenets, S.; Altman, R. Caffeine for treatment of Parkinson disease: A randomized controlled trial. Neurology 2012, 79, 651–658. [Google Scholar] [CrossRef]
- Reichmann, H.J.A.; Diseases, N. Caffeine, chocolate, and adenosine antagonism in Parkinson’s disease. Ageing Neur Dis. 2022, 2, 19. [Google Scholar] [CrossRef]
- Leodori, G.; De Bartolo, M.I.; Belvisi, D.; Ciogli, A.; Fabbrini, A.; Costanzo, M.; Manetto, S.; Conte, A.; Villani, C.; Fabbrini, G.; et al. Salivary caffeine in Parkinson’s disease. Sci. Rep. 2021, 11, 9823. [Google Scholar] [CrossRef]
- Ren, X.; Chen, J.F. Caffeine and Parkinson’s Disease: Multiple Benefits and Emerging Mechanisms. Front. Neurosci. 2020, 14, 602697. [Google Scholar] [CrossRef]
- Altman, R.D.; Lang, A.E.; Postuma, R.B. Caffeine in Parkinson’s disease: A pilot open-label, dose-escalation study. Mov. Disord. 2011, 26, 2427–2431. [Google Scholar] [CrossRef]
- Low, J.J.-L.; Tan, B.J.-W.; Yi, L.-X.; Zhou, Z.-D.; Tan, E.-K. Genetic susceptibility to caffeine intake and metabolism: A systematic review. J. Transl. Med. 2024, 22, 961. [Google Scholar] [CrossRef] [PubMed]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. Caffeine: An Overview of Its Beneficial Effects in Experimental Models and Clinical Trials of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 4766. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ren, X.; Liu, Y.; Li, Z.; Zhang, L.; Chen, X.; He, C.; Chen, J.-F. Aberrant adenosine A2A receptor signaling contributes to neurodegeneration and cognitive impairments in a mouse model of synucleinopathy. Exp. Neurol. 2016, 283, 213–223. [Google Scholar] [CrossRef]
- Jain, R.; Vora, L.; Nathiya, D.; Khatri, D.K. Nrf2–Keap1 Pathway and NLRP3 Inflammasome in Parkinson’s Disease: Mechanistic Crosstalk and Therapeutic Implications. Mol. Neurobiol. 2026, 63, 91. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Xie, S.P.; Saw, W.T.; Ho, P.G.H.; Wang, H.Y.; Zhou, L.; Zhao, Y.; Tan, E.K. The therapeutic implications of tea polyphenols against dopamine (DA) neuron degeneration in Parkinson’s disease (PD). Cells 2019, 8, 911. [Google Scholar] [CrossRef]
- Palacios, N.; Gao, X.; McCullough, M.L.; Schwarzschild, M.A.; Shah, R.; Gapstur, S.; Ascherio, A. Caffeine and risk of Parkinson’s disease in a large cohort of men and women. Mov. Disord. Off. J. Mov. Disord. Soc. 2012, 27, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.T.; Chan, L.; Bai, C.H. The Effect of Caffeine on the Risk and Progression of Parkinson’s Disease: A Meta-Analysis. Nutrients 2020, 12, 1860. [Google Scholar] [CrossRef]
- Ong, Y.L.; Deng, X.; Li, H.H.; Narasimhalu, K.; Chan, L.L.; Prakash, K.M.; Au, W.L.; Ratnagopal, P.; Tan, L.C.S.; Tan, E.K. Caffeine intake interacts with Asian gene variants in Parkinson’s disease: A study in 4488 subjects. Lancet Reg. Health–West. Pac. 2023, 40, 100877. [Google Scholar] [CrossRef]
- Matikainen-Ankney, B.A.; Kezunovic, N.; Menard, C.; Flanigan, M.E.; Zhong, Y.; Russo, S.J.; Benson, D.L.; Huntley, G.W. Parkinson’s disease-linked lrrk2-g2019s mutation alters synaptic plasticity and promotes resilience to chronic social stress in young adulthood. J. Neurosci. 2018, 38, 9700–9711. [Google Scholar] [CrossRef]
- Xu, K.; Xu, Y.-H.; Chen, J.-F.; Schwarzschild, M.A. Neuroprotection by caffeine: Time course and role of its metabolites in the MPTP model of Parkinson’s disease. Neuroscience 2010, 167, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Chen, J.-F.; Uchida, S.; Durlach, C.; King, S.M.; Jenner, P. The Pharmacological Potential of Adenosine A2A Receptor Antagonists for Treating Parkinson’s Disease. Molecules 2022, 27, 2366. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Y.-G.; Chen, J.-F. Targeting the adenosine A2A receptor for neuroprotection and cognitive improvement in traumatic brain injury and Parkinson’s disease. Chin. J. Traumatol. 2024, 27, 125–133. [Google Scholar] [CrossRef]
- Ősz, B.E.; Jîtcă, G.; Ștefănescu, R.E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C.E. Caffeine and Its Antioxidant Properties-It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef]
- Luo, X.; Liu, Y.; Balck, A.; Klein, C.; Fleming, R.M.T. Identification of metabolites reproducibly associated with Parkinson’s Disease via meta-analysis and computational modelling. npj Park. Dis. 2024, 10, 126. [Google Scholar] [CrossRef]
- Liang, Y.; Dai, X.; Cao, Y.; Wang, X.; Lu, J.; Xie, L.; Liu, K.; Li, X. The neuroprotective and antidiabetic effects of trigonelline: A review of signaling pathways and molecular mechanisms. Biochimie 2023, 206, 93–104. [Google Scholar] [CrossRef]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaź, P. Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int. J. Mol. Sci. 2020, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Pharmacological Activities, Therapeutic Effects, and Mechanistic Actions of Trigonelline. Int. J. Mol. Sci. 2024, 25, 3385. [Google Scholar] [CrossRef]
- Khalili, M.; Alavi, M.; Esmaeil-Jamaat, E.; Baluchnejadmojarad, T.; Roghani, M. Trigonelline mitigates lipopolysaccharide-induced learning and memory impairment in the rat due to its anti-oxidative and anti-inflammatory effect. Int. Immunopharmacol. 2018, 61, 355–362. [Google Scholar] [CrossRef]
- Wang, L.; Cai, X.; Shi, M.; Xue, L.; Kuang, S.; Xu, R.; Qi, W.; Li, Y.; Ma, X.; Zhang, R.; et al. Identification and optimization of piperine analogues as neuroprotective agents for the treatment of Parkinson’s disease via the activation of Nrf2/keap1 pathway. Eur. J. Med. Chem. 2020, 199, 112385. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hu, X.; Xu, R.; Zhao, Y.; Xiong, L.; Ai, J.; Wang, X.; Chen, X.; Ba, Y.; Xing, Z.; et al. Piperine promotes PI3K/AKT/mTOR-mediated gut-brain autophagy to degrade α-synuclein in Parkinson’s disease rats. J. Ethnopharmacol. 2024, 322, 117628. [Google Scholar] [CrossRef]
- Li, R.; Lu, Y.; Zhang, Q.; Liu, W.; Yang, R.; Jiao, J.; Liu, J.; Gao, G.; Yang, H. Piperine promotes autophagy flux by P2RX4 activation in SNCA/α-synuclein-induced Parkinson disease model. Autophagy 2022, 18, 559–575. [Google Scholar] [CrossRef]
- Liu, J.; Chen, M.; Wang, X.; Wang, Y.; Duan, C.; Gao, G.; Lu, L.; Wu, X.; Wang, X.; Yang, H. Piperine induces autophagy by enhancing protein phosphotase 2A activity in a rotenone-induced Parkinson’s disease model. Oncotarget 2016, 7, 60823. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Azam, S.; Kim, I.-S.; Choi, D.-K. Neuroprotective Effects of Black Pepper and Its Bioactive Compounds in Age-Related Neurological Disorders. Aging Dis. 2023, 14, 750–777. [Google Scholar] [CrossRef]
- Sharma, S.; Raj, K.; Singh, S. Neuroprotective Effect of Quercetin in Combination with Piperine Against Rotenone- and Iron Supplement-Induced Parkinson’s Disease in Experimental Rats. Neurotox. Res. 2020, 37, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ma, L.; Racette, S.B.; Anderson Spearie, C.L.; Ostlund, R.E., Jr. Phytosterol glycosides reduce cholesterol absorption in humans. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 296, G931–G935. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, F.; Shen, M.; Jia, S.; Xie, J. Phytosterols suppress phagocytosis and inhibit inflammatory mediators via ERK pathway on LPS-triggered inflammatory responses in RAW264. 7 macrophages and the correlation with their structure. Foods 2019, 8, 582. [Google Scholar] [CrossRef]
- Cui, X.; Lin, Q.; Liang, Y. Plant-derived antioxidants protect the nervous system from aging by inhibiting oxidative stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Armand, E.F.; Shantaram, M.; Nico, N.F.; Simon, F.N.; Paul, M.F. Potential of medicinal plant compounds to targeting Tau protein in the therapy of Alzheimer’s disease—A review. Biomedicine 2019, 39, 217–227. [Google Scholar] [CrossRef]
- Guo, X.; Yu, J.; Wang, R.; Peng, N.; Li, R. Deciphering the effect of phytosterols on Alzheimer’s disease and Parkinson’s disease: The mediating role of lipid profiles. Alzheimer’s Res. Ther. 2024, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rojas, L.O.; Martínez-Dávila, I.A.; Luna-Herrera, C.; Gutierrez-Castillo, M.E.; Lopez-Salas, F.E.; Gatica-Garcia, B.; Soto-Rodriguez, G.; Bringas Tobon, M.E.; Flores, G.; Padilla-Viveros, A.; et al. Unilateral intranigral administration of β-sitosterol β-D-glucoside triggers pathological α-synuclein spreading and bilateral nigrostriatal dopaminergic neurodegeneration in the rat. Acta Neuropathol. Commun. 2020, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rojas, L.O.; Garces-Ramirez, L.; Luna-Herrera, C.; Flores-Martinez, Y.M.; Soto-Rodriguez, G.; Gatica-Garcia, B.; Lopez-Salas, F.E.; Ayala-Davila, J.; Gutierrez-Castillo, M.E.; Padilla-Viveros, A.; et al. A single intranigral administration of β-sitosterol β-d-glucoside elicits bilateral sensorimotor and non-motor alterations in the rat. Behav. Brain Res. 2020, 378, 112279. [Google Scholar] [CrossRef]
- Bigelow, L.J.; Perry, M.A.; Ogilvie, S.L.; Tasker, R.A. Longitudinal Assessment of Behaviour and Associated Bio-Markers Following Chronic Consumption of β-Sitosterol β-D-Glucoside in Rats: A Putative Model of Parkinson’s Disease. Front. Neurosci. 2022, 16, 810148. [Google Scholar] [CrossRef]
- Smith, L.; Schapira, A.H.V. GBA Variants and Parkinson Disease: Mechanisms and Treatments. Cells 2022, 11, 1261. [Google Scholar] [CrossRef]
- Smith, L.J.; Lee, C.Y.; Menozzi, E.; Schapira, A.H.V. Genetic variations in GBA1 and LRRK2 genes: Biochemical and clinical consequences in Parkinson disease. Front. Neurol. 2022, 13, 971252. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.P.; Kim, D.; Kim, S.; Kim, S.; Karuppagounder, S.S.; Kwon, S.-H.; Lee, S.; Kam, T.-I.; Lee, S.; Ham, S.; et al. α-Synuclein accumulation and GBA deficiency due to L444P GBA mutation contributes to MPTP-induced parkinsonism. Mol. Neurodegener. 2018, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Marek, K.; Chowdhury, S.; Siderowf, A.; Lasch, S.; Coffey, C.S.; Caspell-Garcia, C.; Simuni, T.; Jennings, D.; Tanner, C.M.; Trojanowski, J.Q.; et al. The Parkinson’s progression markers initiative (PPMI)—Establishing a PD biomarker cohort. Ann. Clin. Transl. Neurol. 2018, 5, 1460–1477. [Google Scholar] [CrossRef]

| Name | Internal Standard | Q1 m/z | Q3 m/z | CE (V) | % Samples with Missing Area Ratio |
|---|---|---|---|---|---|
| Caffeine | Niacinamide-d4 | 195.1 | 138 | 20 | 0.1 |
| Paraxanthine | Niacinamide-d4 | 181.1 | 124 | 24 | 0.1 |
| Trigonelline | Methionine-d3 | 138.1 | 94.1 | 20 | 0 |
| Piperine | Niacinamide-d4 | 286.1 | 201.1 | 20 | 0.1 |
| Sitosteryl hexoside | CE (18:1(d7)) | 594.6 | 397.4 | 17 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Dong, X.; Zheng, Y.; Tan, E.T.Y.; Sun, Q.Y.; Xiao, B.; Tan, E.K.; Wu, Y.-C.; Zhou, Z.D. Plasma Levels of Food-Derived Metabolites as Biomarkers of Parkinson’s Disease. Int. J. Mol. Sci. 2026, 27, 16. https://doi.org/10.3390/ijms27010016
Dong X, Zheng Y, Tan ETY, Sun QY, Xiao B, Tan EK, Wu Y-C, Zhou ZD. Plasma Levels of Food-Derived Metabolites as Biomarkers of Parkinson’s Disease. International Journal of Molecular Sciences. 2026; 27(1):16. https://doi.org/10.3390/ijms27010016
Chicago/Turabian StyleDong, Xiaoxue, Yilong Zheng, Evelyn Ting Ying Tan, Qiao Yang Sun, Bin Xiao, Eng King Tan, Yun-Cheng Wu, and Zhi Dong Zhou. 2026. "Plasma Levels of Food-Derived Metabolites as Biomarkers of Parkinson’s Disease" International Journal of Molecular Sciences 27, no. 1: 16. https://doi.org/10.3390/ijms27010016
APA StyleDong, X., Zheng, Y., Tan, E. T. Y., Sun, Q. Y., Xiao, B., Tan, E. K., Wu, Y.-C., & Zhou, Z. D. (2026). Plasma Levels of Food-Derived Metabolites as Biomarkers of Parkinson’s Disease. International Journal of Molecular Sciences, 27(1), 16. https://doi.org/10.3390/ijms27010016







