Transcriptomic Plasticity of Human Alveolar Macrophages Revealed by Single-Cell RNA Sequencing Following Drug Exposure: Implications for Therapeutic Development
Abstract
1. Introduction
2. Results
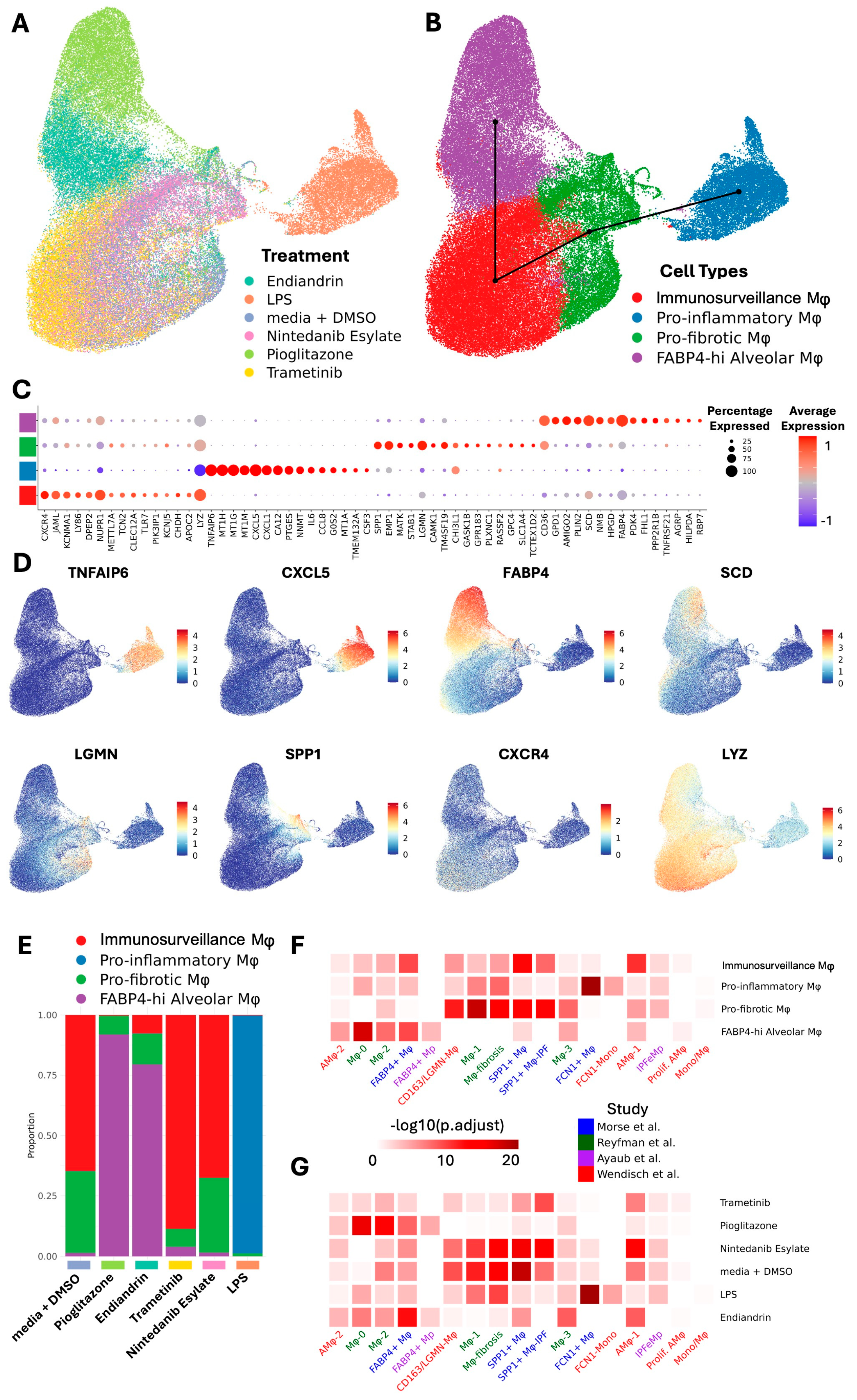
2.1. Human Lung Macrophage Transcriptomics Are Globally Plastic and Amenable to Pharmacological Modulation
2.2. AM Transcriptional Reprogramming Occurs at the Transcriptional Level, Is Predictable, and Allows a Change in Functionality
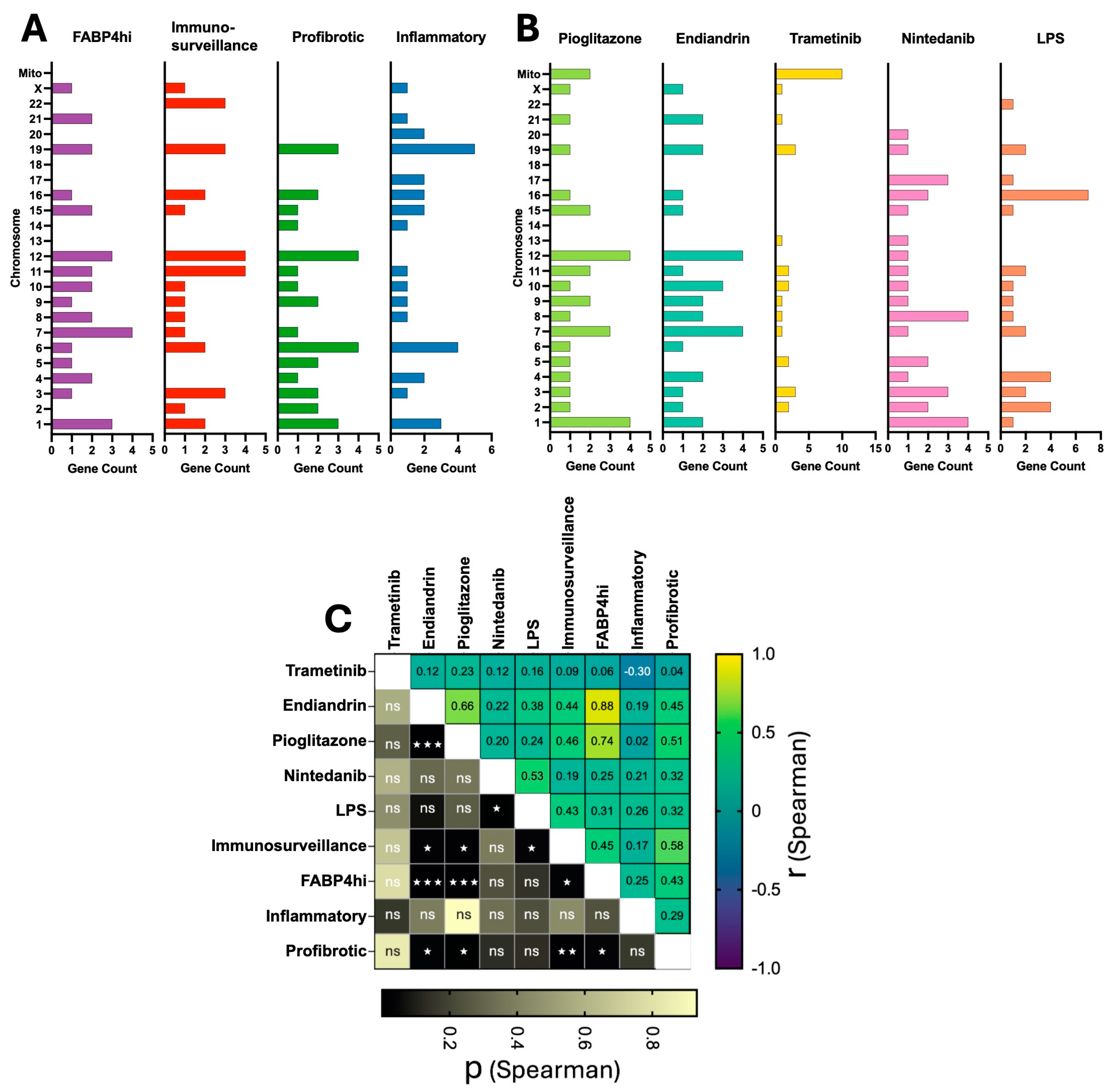
2.3. Plasticity in AM Transcriptomes Is Evident at the Chromosome Level
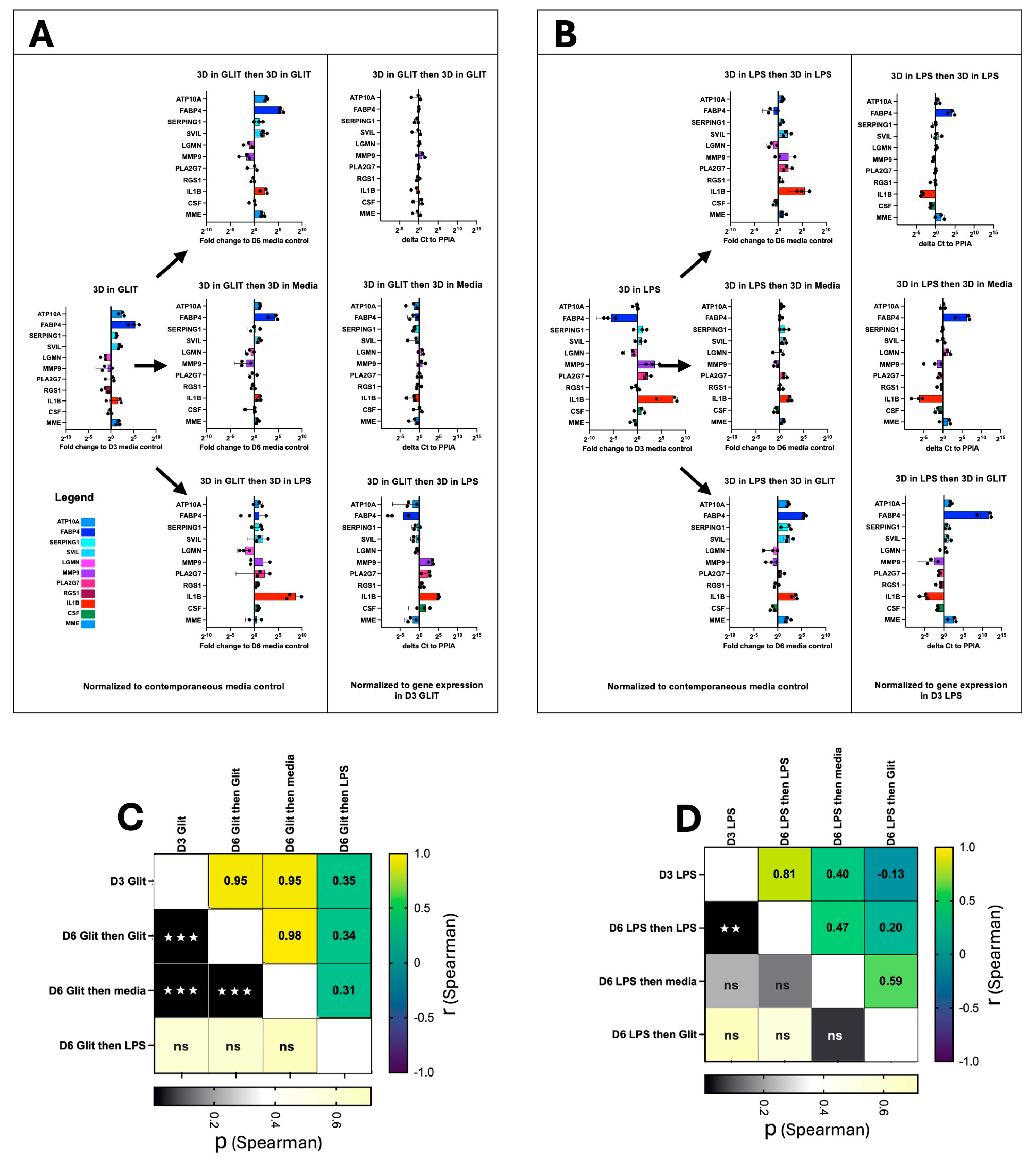
2.4. AM Transcriptional Plasticity Remains Following Drug Perturbation
3. Discussion
4. Materials and Methods
4.1. Collection and Cryopreservation of Human AM
4.2. Cell Culture Conditions
4.3. Preparation of Cells for Performing scRNA-Seq
4.4. scRNA-Seq
4.5. scRNA-Seq Bioinformatic Analysis
4.6. Cell Culture for Experiments Assessing Plasticity Following Serial Compound Treatment
4.7. Quantitative PCR Analysis
4.8. Chromosome Location
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olmeda, B.; Martínez-Calle, M.; Pérez-Gil, J. Pulmonary surfactant metabolism in the alveolar airspace: Biogenesis, extracellular conversions, recycling. Ann. Anat. Anat. Anz. 2017, 209, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Terada, H. Chapter 5—Particle-manufacturing technology-based inhalation therapy for pulmonary diseases. In Colloid and Interface Science in Pharmaceutical Research and Development; Ohshima, H., Makino, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 103–119. [Google Scholar] [CrossRef]
- Wright, J.R.; Clements, J.A. Metabolism and Turnover of Lung Surfactant. Am. Rev. Respir. Dis. 1987, 136, 426–444. [Google Scholar] [CrossRef]
- Divithotawela, C.; Apte, S.H.; Tan, M.E.; De Silva, T.A.; Chambers, D.C. Pulmonary alveolar proteinosis after lung transplantation. Respirol. Case Rep. 2020, 8, e00566. [Google Scholar] [CrossRef] [PubMed]
- Twigg, H.L. 3rd. Macrophages in innate and acquired immunity. Semin. Respir. Crit. Care Med. 2004, 25, 21–31. [Google Scholar] [CrossRef]
- Zheng, J.; Li, Y.; Kong, X.; Guo, J. Exploring immune-related pathogenesis in lung injury: Providing new insights Into ALI/ARDS. Biomed. Pharmacother. 2024, 175, 116773. [Google Scholar] [CrossRef] [PubMed]
- Adler, M.; Mayo, A.; Zhou, X.; Franklin, R.A.; Meizlish, M.L.; Medzhitov, R.; Kallenberger, S.M.; Alon, U. Principles of Cell Circuits for Tissue Repair and Fibrosis. iScience 2020, 23, 100841. [Google Scholar] [CrossRef]
- Alrajhi, N.N. Post-COVID-19 pulmonary fibrosis: An ongoing concern. Ann. Thorac. Med. 2023, 18, 173–181. [Google Scholar] [CrossRef]
- Adams, T.S.; Schupp, J.C.; Poli, S.; Ayaub, E.A.; Neumark, N.; Ahangari, F.; Chu, S.G.; Raby, B.A.; DeIuliis, G.; Januszyk, M.; et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1983. [Google Scholar] [CrossRef]
- Aran, D.; Looney, A.P.; Liu, L.; Wu, E.; Fong, V.; Hsu, A.; Chak, S.; Naikawadi, R.P.; Wolters, P.J.; Abate, A.R.; et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019, 20, 163–172. [Google Scholar] [CrossRef]
- Morse, C.; Tabib, T.; Sembrat, J.; Buschur, K.L.; Bittar, H.T.; Valenzi, E.; Jiang, Y.; Kass, D.J.; Gibson, K.; Chen, W.; et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur. Respir. J. 2019, 54, 1802441. [Google Scholar] [CrossRef]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.-I.; Ren, Z.; et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 199, 1517–1536. [Google Scholar] [CrossRef]
- Ayaub, E.A.; Poli, S.; Ng, J.; Adams, T.; Schupp, J.; Quesada-Arias, L.; Poli, F.; Cosme, C.; Robertson, M.; Martinez-Manzano, J.; et al. Single Cell RNA-seq and Mass Cytometry Reveals a Novel and a Targetable Population of Macrophages in Idiopathic Pulmonary Fibrosis. bioRxiv 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.01.04.425268v1.full (accessed on 27 February 2025).
- Wendisch, D.; Dietrich, O.; Mari, T.; von Stillfried, S.; Ibarra, I.L.; Mittermaier, M.; Mache, C.; Chua, R.L.; Knoll, R.; Timm, S.; et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell 2021, 184, 6243–6261.e27. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.I.; Puritz, C.H.; Senkow, K.J.; Markov, N.S.; Diaz, E.; Jonasson, E.; Yu, Z.; Swaminathan, S.; Lu, Z.; Fenske, S.; et al. Profibrotic monocyte-derived alveolar macrophages are expanded in patients with persistent respiratory symptoms and radiographic abnormalities after COVID-19. Nat. Immunol. 2024, 25, 2097–2109. [Google Scholar] [CrossRef]
- Zhang, F.; Ayaub, E.A.; Wang, B.; Puchulu-Campanella, E.; Li, Y.H.; Hettiarachchi, S.U.; Lindeman, S.D.; Luo, Q.; Rout, S.; Srinivasarao, M.; et al. Reprogramming of profibrotic macrophages for treatment of bleomycin-induced pulmonary fibrosis. EMBO Mol. Med. 2020, 12, e12034. [Google Scholar] [CrossRef]
- Singh, A.; Chakraborty, S.; Wong, S.W.; Hefner, N.A.; Stuart, A.; Qadir, A.S.; Mukhopadhyay, A.; Bachmaier, K.; Shin, J.W.; Rehman, J.; et al. Nanoparticle targeting of de novo profibrotic macrophages mitigates lung fibrosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2121098119. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Chang, W.-H.; Linderholm, A.; Yang, D.C.; Liu, S.A.; Hsu, S.-W.; Chen, C.-H. Upregulation of MARCKS in Macrophage Reprogramming and Its Potential as a Therapeutic Target for Pulmonary Fibrosis [abstract]. Am. J. Respir. Crit. Care Med. 2024, 209, A3236. [Google Scholar] [CrossRef]
- Isshiki, T.; Vierhout, M.; Naiel, S.; Ali, P.; Yazdanshenas, P.; Kumaran, V.; Yang, Z.; Dvorkin-Gheva, A.; Rullo, A.F.; Kolb, M.R.J.; et al. Therapeutic strategies targeting pro-fibrotic macrophages in interstitial lung disease. Biochem. Pharmacol. 2023, 211, 115501. [Google Scholar] [CrossRef]
- Apte, S.H.; Groves, P.L.; Tan, M.E.; Lutzky, V.P.; de Silva, T.; Monteith, J.N.; Yerkovich, S.T.; O’Sullivan, B.J.; Davis, R.A.; Chambers, D.C. A Methodological Approach to Identify Natural Compounds with Antifibrotic Activity and the Potential to Treat Pulmonary Fibrosis Using Single-Cell Sequencing and Primary Human Lung Macrophages. Int. J. Mol. Sci. 2023, 24, 15104. [Google Scholar] [CrossRef]
- Chambers, D.C.; Apte, S.H.; Deller, D.; Masel, P.J.; Jones, C.M.; Newbigin, K.; Matula, M.; Rapchuk, I.L. Radiological outcomes of whole lung lavage for artificial stone-associated silicosis. Respirology 2021, 26, 501–503. [Google Scholar] [CrossRef]
- Davis, R.A.; Carroll, A.R.; Duffy, S.; Avery, V.M.; Guymer, G.P.; Forster, P.I.; Quinn, R.J. Endiandrin A, a potent glucocorticoid receptor binder isolated from the Australian plant Endiandra anthropophagorum. J. Nat. Prod. 2007, 70, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Street, K.; Risso, D.; Fletcher, R.B.; Das, D.; Ngai, J.; Yosef, N.; Purdom, E.; Dudoit, S. Slingshot: Cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genom. 2018, 19, 477. [Google Scholar] [CrossRef]
- Warfel, A.H.; Zucker-Franklin, D. Down-regulation of macrophage lysozyme by lipopolysaccharide and interferon. J. Immunol. 1986, 137, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.-S.; Shaw, N.S.; Vinckenbosch, N.; Liu, P.; Yasmin, R.; Desvergne, B.; Wahli, W.; Noy, N. Selective Cooperation between Fatty Acid Binding Proteins and Peroxisome Proliferator-Activated Receptors in Regulating Transcription. Mol. Cell. Biol. 2002, 22, 5114–5127. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Q.; Liang, W.; Yan, R.; Tong, L.; Jia, M.; Zhao, C.; Zhao, W. TRIM28 SUMOylates and stabilizes NLRP3 to facilitate inflammasome activation. Nat. Commun. 2021, 12, 4794. [Google Scholar] [CrossRef]
- Becerra-Diaz, M.; Song, M.; Heller, N. Androgen and Androgen Receptors as Regulators of Monocyte and Macrophage Biology in the Healthy and Diseased Lung. Front. Immunol. 2020, 11, 1698. [Google Scholar] [CrossRef]
- Cruz, C.M.; Rinna, A.; Forman, H.J.; Ventura, A.L.M.; Persechini, P.M.; Ojcius, D.M. ATP Activates a Reactive Oxygen Species-dependent Oxidative Stress Response and Secretion of Proinflammatory Cytokines in Macrophages*. J. Biol. Chem. 2007, 282, 2871–2879. [Google Scholar] [CrossRef]
- Tang, J.X.; Thompson, K.; Taylor, R.W.; Oláhová, M. Mitochondrial OXPHOS Biogenesis: Co-Regulation of Protein Synthesis, Import, and Assembly Pathways. Int. J. Mol. Sci. 2020, 21, 3820. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lian, Z.; Xia, X.; Lu, Y.; Hu, K.; Zhang, Y.; Liu, Y.; Hu, L.; Yuan, K.; Sun, Z.; et al. Targeting metabolic vulnerability in mitochondria conquers MEK inhibitor resistance in KRAS-mutant lung cancer. Acta Pharm. Sin. B 2023, 13, 1145–1163. [Google Scholar] [CrossRef]
- Byrne, A.J.; Powell, J.E.; O’Sullivan, B.J.; Ogger, P.P.; Hoffland, A.; Cook, J.; Bonner, K.L.; Hewitt, R.J.; Wolf, S.; Ghai, P.; et al. Dynamics of human monocytes and airway macrophages during healthy aging and after transplant. J. Exp. Med. 2020, 217, e20191236. [Google Scholar] [CrossRef] [PubMed]
- Pereverzeva, L.; van Linge, C.C.A.; Schuurman, A.R.; Klarenbeek, A.M.; Ramirez Moral, I.; Otto, N.A.; Peters-Sengers, H.; Butler, J.M.; Schomakers, B.V.; van Weeghel, M.; et al. Human alveolar macrophages do not rely on glucose metabolism upon activation by lipopolysaccharide. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166488. [Google Scholar] [CrossRef]
- Osorio, D.; Cai, J.J. Systematic determination of the mitochondrial proportion in human and mice tissues for single-cell RNA-sequencing data quality control. Bioinformatics 2021, 37, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M.; Annane, D. Compartmentalization of the inflammatory response in sepsis and SIRS. J. Endotoxin Res. 2006, 12, 151–170. [Google Scholar] [CrossRef]
- Brooks, D.; Barr, L.C.; Wiscombe, S.; McAuley, D.F.; Simpson, A.J.; Rostron, A.J. Human lipopolysaccharide models provide mechanistic and therapeutic insights into systemic and pulmonary inflammation. Eur. Respir. J. 2020, 56, 1901298. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto Natasja, A.; van der Velden, S.; Neele Annette, E.; van den Berg Susan, M.; Luque-Martin, R.; Chen, H.-J.; Boshuizen Marieke, C.S.; Ahmed, M.; et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef]
- Zulfiqar, B.; Jones, A.J.; Sykes, M.L.; Shelper, T.B.; Davis, R.A.; Avery, V.M. Screening a Natural Product-Based Library against Kinetoplastid Parasites. Molecules 2017, 22, 1715. [Google Scholar] [CrossRef]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- HGNC. Available online: https://www.genenames.org/tools/multi-symbol-checker/ (accessed on 27 February 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groves, P.L.; Hockey, L.; O’Sullivan, B.J.; Zhang, L.-Y.; Xiong, Z.; Nguyen, Q.H.; Tan, M.E.; Lutzky, V.P.; Davis, R.A.; Chambers, D.C.; et al. Transcriptomic Plasticity of Human Alveolar Macrophages Revealed by Single-Cell RNA Sequencing Following Drug Exposure: Implications for Therapeutic Development. Int. J. Mol. Sci. 2025, 26, 4439. https://doi.org/10.3390/ijms26094439
Groves PL, Hockey L, O’Sullivan BJ, Zhang L-Y, Xiong Z, Nguyen QH, Tan ME, Lutzky VP, Davis RA, Chambers DC, et al. Transcriptomic Plasticity of Human Alveolar Macrophages Revealed by Single-Cell RNA Sequencing Following Drug Exposure: Implications for Therapeutic Development. International Journal of Molecular Sciences. 2025; 26(9):4439. https://doi.org/10.3390/ijms26094439
Chicago/Turabian StyleGroves, Penny L., Levi Hockey, Brendan J. O’Sullivan, Lai-Ying Zhang, Zherui Xiong, Quan H. Nguyen, Maxine E. Tan, Viviana P. Lutzky, Rohan A. Davis, Daniel C. Chambers, and et al. 2025. "Transcriptomic Plasticity of Human Alveolar Macrophages Revealed by Single-Cell RNA Sequencing Following Drug Exposure: Implications for Therapeutic Development" International Journal of Molecular Sciences 26, no. 9: 4439. https://doi.org/10.3390/ijms26094439
APA StyleGroves, P. L., Hockey, L., O’Sullivan, B. J., Zhang, L.-Y., Xiong, Z., Nguyen, Q. H., Tan, M. E., Lutzky, V. P., Davis, R. A., Chambers, D. C., & Apte, S. H. (2025). Transcriptomic Plasticity of Human Alveolar Macrophages Revealed by Single-Cell RNA Sequencing Following Drug Exposure: Implications for Therapeutic Development. International Journal of Molecular Sciences, 26(9), 4439. https://doi.org/10.3390/ijms26094439






