Deficient Mismatch Repair and Microsatellite Instability in Solid Tumors
Abstract
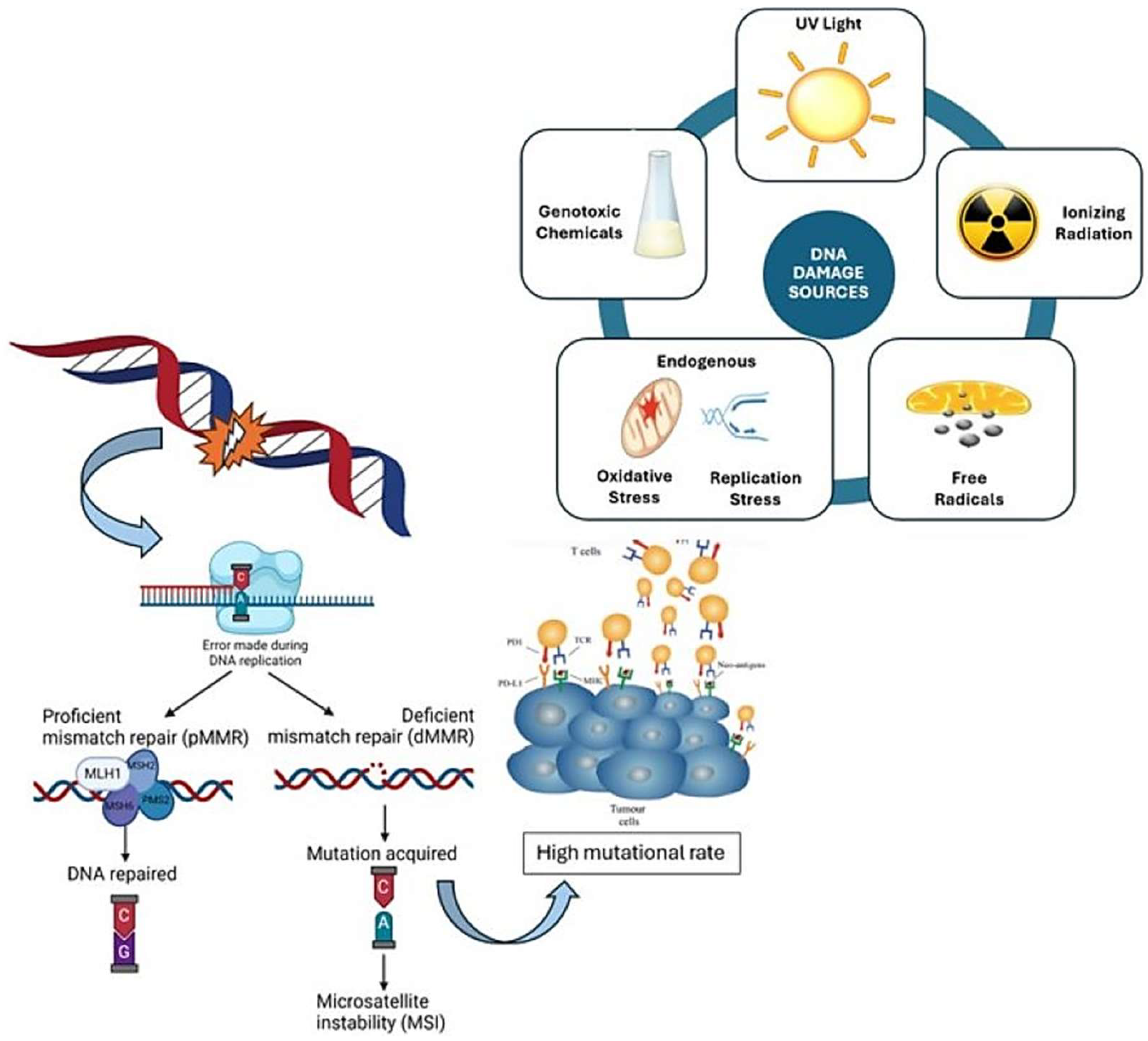
1. Introduction
2. The Landscape of Deficient MMR and MSI in Solid Tumors
2.1. Colorectal Cancers
2.1.1. Lynch Syndrome
2.1.2. MUTYH-Associated Polyposis
2.1.3. Polymerase Proofreading-Associated Polyposis
2.1.4. Sporadic Colorectal Cancers
2.2. Endometrial Cancer
2.3. Gastric Cancer
2.4. Urothelial Carcinoma
2.5. Less Common Neoplasms
3. Overview of Therapeutic Approaches in the Treatment of dMMR/MSI-H Solid Tumors
3.1. Immunotherapy
3.1.1. Immunotherapy Use in Early-Stage Disease
3.1.2. Immunotherapy in the Metastatic Setting
3.1.3. The Challenge of Resistance to Immunotherapy
3.2. The Impact of dMMR/MSI-H Status on Chemotherapy Efficacy and Outcomes
4. Conclusions and Future Perspectives Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| BER | Base excision repair |
| CIMP | CpG island methylator phenotype |
| CIN | Chromosomal instability |
| CRC | Colorectal cancer |
| DFS | Disease-free survival |
| dMMR | Deficient mismatch repair |
| EC | Endometrial cancer |
| EPCAM | Epithelial cellular adhesion molecule |
| FDA | Food and Drug Administration |
| FLOT | Fluorouracil, leucovorin, oxaliplatin, and docetaxel |
| FMT | Fecal microbiota transplantation |
| FOLFOX | Folinic acid (leucovorin), fluorouracil, and oxaliplatin |
| GC | Gastric cancer |
| GEJ | Gastroesophageal junction |
| HLA | Human leukocyte antigen |
| HNPCC | Hereditary non-polyposis |
| ICI | Immune checkpoint inhibitor |
| IDLs | Insertion–deletion loops |
| IHC | Immunohistochemistry |
| LOH | Loss of heterozygosity |
| LS | Lynch syndrome |
| LS-CRC | Lynch syndrome-associated colorectal cancer |
| LVI | Lymphovascular invasion |
| MAP M | UTYH-associated polyposis |
| MMR | Mismatch repair |
| MSI | Microsatellite instability |
| MSI-H | Microsatellite instability high |
| MSS | Microsatellite stable/microsatellite stability |
| NCCN | National Comprehensive Cancer Network |
| OS | Overall survival |
| ORR | Overall response rate |
| PCR | Polymerase chain reaction |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| PFS | Progression-free survival |
| pMMR | Proficient mismatch repair |
| PPAP | Polymerase proofreading-associated polyposis |
| TCGA | The Cancer Genome Atlas |
| TILs | Tumor-infiltrating lymphocytes |
| TMB | Tumor mutational burden |
| UC | Urothelial cancer |
References
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Ellegren, H. Microsatellites: Simple sequences with complex evolution. Nat. Rev. Genet. 2004, 5, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Li, G.M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Jiricny, J. The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 2006, 7, 335–346. [Google Scholar] [CrossRef]
- Peltomaki, P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J. Clin. Oncol. 2003, 21, 1174–1179. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y.; Wang, C. Beyond Tumor Mutation Burden: Tumor Neoantigen Burden as a Biomarker for Immunotherapy and Other Types of Therapy. Front. Oncol. 2021, 11, 672677. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, J.; Chu, Q.; Duan, J.; Zhang, J.; Bai, H.; Yang, Z.; Fang, W.; Cai, L.; Wan, R.; et al. A Novel Tumor Mutational Burden Estimation Model as a Predictive and Prognostic Biomarker in NSCLC Patients. BMC Med. 2020, 18, 232. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the Use of Next-Generation Sequencing (NGS) for Patients with Metastatic Cancers: A Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Ward, J.P.; Gubin, M.M.; Schreiber, R.D. The Role of Neoantigens in Naturally Occurring and Therapeutically Induced Immune Responses to Cancer. Adv. Immunol. 2016, 130, 25–74. [Google Scholar]
- McConechy, M.K.; Talhouk, A.; Li-Chang, H.H.; Leung, S.; Huntsman, D.G.; Gilks, C.B.; McAlpine, J.N. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol. Oncol. 2015, 137, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Pastor, D.M.; Schlom, J. Immunology of Lynch Syndrome. Curr. Oncol. Rep. 2021, 23, 96. [Google Scholar] [CrossRef] [PubMed]
- Kravochuck, S.E.; Kalady, M.F.; Burke, C.A.; Heald, B.; Church, J.M. Defining HNPCC and Lynch syndrome: What’s in a name? Gut 2014, 63, 1525–1526. [Google Scholar] [CrossRef] [PubMed]
- Jass, J. Hereditary non-polyposis colorectal cancer: The rise and fall of a confusing term. World J. Gastroenterol. 2006, 12, 4943–4950. [Google Scholar] [CrossRef]
- Vasen, H.F.; Watson, P.; Mecklin, J.P.; Lynch, H.T. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999, 116, 1453–1456. [Google Scholar] [CrossRef]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; de la Chapelle, A.; Ruschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef]
- Drescher, K.M.; Sharma, P.; Lynch, H.T. Current hypotheses on how microsatellite instability leads to enhanced survival of Lynch syndrome patients. J. Immunol. Res. 2010, 2010, 170432. [Google Scholar] [CrossRef]
- Zetner, D.B.; Bisgaard, M.L. Familial colorectal cancer type X. Curr. Genom. 2017, 18, 341–359. [Google Scholar] [CrossRef][Green Version]
- Mueller-Koch, Y.; Vogelsang, H.; Kopp, R.; Lohse, P.; Keller, G.; Aust, D.; Muders, M.; Gross, M.; Daum, J.; Schiemann, U.; et al. Hereditary non-polyposis colorectal cancer: Clinical and molecular evidence for a new entity of hereditary colorectal cancer. Gut 2005, 54, 1733–1740. [Google Scholar] [CrossRef]
- Lynch, H.T.; Lanspa, S.; Smyrk, T.; Boman, B.; Watson, P.; Lynch, J. Hereditary nonpolyposis colorectal cancer (Lynch syndromes I & II). Genetics, pathology, natural history, and cancer control, Part, I. Cancer Genet. Cytogenet. 1991, 53, 143–160. [Google Scholar]
- Carethers, J.M.; Stoffel, E.M. Lynch syndrome and Lynch syndrome mimics: The growing complex landscape of hereditary colon cancer. World J. Gastroenterol. 2015, 21, 9253–9261. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.C.; Valentin, M.D.; Ferreira, F.d.O.; Carraro, D.M.; Rossi, B.M. Mismatch repair genes in Lynch syndrome: A review. Sao Paulo Med. J. 2009, 127, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007, 50, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Kinzler, K.W.; Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 1996, 87, 159–170. [Google Scholar] [CrossRef]
- Eide, T.J. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int. J. Cancer 1986, 38, 173–176. [Google Scholar] [CrossRef]
- Vasen, H.F.; Mecklin, J.P.; Khan, P.M.; Lynch, H.T. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis. Colon Rectum 1991, 34, 424–425. [Google Scholar] [CrossRef]
- Newton, K.; Green, K.; Lalloo, F.; Evans, D.G.; Hill, J. Colonoscopy screening compliance and outcomes in patients with Lynch syndrome. Color. Dis. 2015, 17, 38–46. [Google Scholar] [CrossRef]
- Steinke, V.; Engel, C.; Büttner, R.; Schackert, H.K.; Schmiegel, W.H.; Propping, P. Hereditary nonpolyposis colorectal cancer (HNPCC)/Lynch syndrome. Dtsch. Ärzteblatt Int. 2013, 110, 32–38. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Papadopoulos, N.; Liu, B.; Wei, Y.F.; Carter, K.C.; Ruben, S.M.; Rosen, C.A.; Haseltine, W.A.; Fleischmann, R.D.; Fraser, C.M.; et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 1994, 371, 75–80. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Nicolaides, N.C.; Wei, Y.F.; Ruben, S.M.; Carter, K.C.; Rosen, C.A.; Haseltine, W.A.; Fleischmann, R.D.; Fraser, C.M.; Adams, M.D.; et al. Mutation of a mutL homolog in hereditary colon cancer. Science 1994, 263, 1625–1629. [Google Scholar] [CrossRef]
- Aarnio, M.; Mecklin, J.P.; Aaltonen, L.A.; Nystrom-Lahti, M.; Jarvinen, H.J. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int. J. Cancer 1995, 64, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Win, A.K.; Lindor, N.M.; Young, J.P.; Macrae, F.A.; Young, G.P.; Williamson, E.; Parry, S.; Goldblatt, J.; Lipton, L.; Winship, I.; et al. Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J. Natl. Cancer Inst. 2012, 104, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Kempers, M.J.; Kuiper, R.P.; Ockeloen, C.W.; Chappuis, P.O.; Hutter, P.; Rahner, N.; Schackert, H.K.; Steinke, V.; Holinski-Feder, E.; Morak, M.; et al. Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: A cohort study. Lancet Oncol. 2011, 12, 49–55. [Google Scholar] [CrossRef]
- Rumilla, K.; Schowalter, K.V.; Lindor, N.M.; Thomas, B.C.; Mensink, K.A.; Gallinger, S.; Holter, S.; Newcomb, P.A.; Potter, J.D.; Jenkins, M.A.; et al. Frequency of deletions of EPCAM (TACSTD1) in MSH2-associated Lynch syndrome cases. J. Mol. Diagn. 2011, 13, 93–99. [Google Scholar] [CrossRef]
- Stoffel, E.M.; Mangu, P.B.; Limburg, P.J.; American Society of Clinical Oncology; European Society for Medical Oncology. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology clinical practice guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology clinical practice guidelines. J. Oncol. Pract. 2015, 11, e437–e441. [Google Scholar] [CrossRef]
- Vangala, D.B.; Cauchin, E.; Balmaña, J.; Wyrwicz, L.; van Cutsem, E.; Güller, U.; Castells, A.; Carneiro, F.; Hammel, P.; Ducreux, M. Screening and surveillance in hereditary gastrointestinal cancers: Recommendations from the European Society of Digestive Oncology (ESDO) expert discussion at the 20th European Society for Medical Oncology (ESMO)/World Congress on Gastrointestinal Cancer, Barcelona, June 2018. Eur. J. Cancer 2018, 104, 91–103. [Google Scholar]
- Stjepanovic, N.; Moreira, L.; Carneiro, F.; Balaguer, F.; Cervantes, A.; Balmaña, J.; Martinelli, E.; ESMO Guidelines Committee. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1558–1571. [Google Scholar] [CrossRef]
- Syngal, S.; Brand, R.E.; Church, J.M.; Giardiello, F.M.; Hampel, H.L.; Burt, R.W.; American College of Gastroenterology. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am. J. Gastroenterol. 2015, 110, 223–262. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Allen, J.I.; Axilbund, J.E.; Boland, C.R.; Burke, C.A.; Burt, R.W.; Church, J.M.; Dominitz, J.A.; Johnson, D.A.; Kaltenbach, T.; et al. Guidelines on Genetic Evaluation and Management of Lynch Syndrome: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2014, 147, 502–526. [Google Scholar] [CrossRef]
- Theodoratou, E.; Campbell, H.; Tenesa, A.; Houlston, R.; Webb, E.; Lubbe, S.; Broderick, P.; Gallinger, S.; Croitoru, E.M.; Jenkins, M.A.; et al. A large-scale meta-analysis to refine colorectal cancer risk estimates associated with MUTYH variants. Br. J. Cancer 2010, 103, 1875–1884. [Google Scholar] [CrossRef]
- Al-Tassan, N.; Chmiel, N.H.; Maynard, J.; Fleming, N.; Livingston, A.L.; Williams, G.T.; Hodges, A.K.; Davies, D.R.; David, S.S.; Sampson, J.R.; et al. Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors. Nat. Genet. 2002, 30, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Viel, A.; Bruselles, A.; Meccia, E.; Fornasarig, M.; Quaia, M.; Canzonieri, V.; Policicchio, E.; Urso, E.D.; Agostini, M.; Genuardi, M.; et al. A specific mutational signature associated with DNA 8-oxoguanine persistence in MUTYH-defective colorectal cancer. EBioMedicine 2017, 20, 39–49. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, N.F.C.C.; Nielsen, M.; Pereira, D.; van Puijenbroek, M.; Vasen, H.F.; Hes, F.J.; van Wezel, T.; Morreau, H. MUTYH-associated polyposis carcinomas frequently lose HLA class I expression—A common event amongst DNA-repair-deficient colorectal cancers. J. Pathol. 2009, 219, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Dierssen, J.W.F.; de Miranda, N.F.C.C.; Mulder, A.; Van Puijenbroek, M.; Verduyn, W.; Claas, F.H.J.; van de Velde, C.J.H.; Fleuren, G.J.; Cornelisse, C.; Corver, W.E.; et al. High-resolution analysis of HLA class I alterations in colorectal cancer. BMC Cancer 2006, 6, 233. [Google Scholar] [CrossRef]
- Dierssen, J.W.F.; de Miranda, N.F.C.C.; Ferrone, S.; van Puijenbroek, M.; Cornelisse, C.J.; Fleuren, G.J.; van Wezel, T.; Morreau, H. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer 2007, 7, 33. [Google Scholar] [CrossRef]
- Kloor, M.; Becker, C.; Benner, A.; Woerner, S.M.; Gebert, J.; Ferrone, S.; von Knebel Doeberitz, M. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005, 65, 6418–6424. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Cleary, S.P.; Cotterchio, M.; Jenkins, M.A.; Kim, H.; Bristow, R.; Green, R.; Haile, R.; Hopper, J.L.; LeMarchand, L.; Lindor, L.; et al. Germline MutY human homologue mutations and colorectal cancer: A multisite case-control study. Gastroenterology 2009, 136, 1251–1260. [Google Scholar] [CrossRef]
- Middeldorp, A.; van Puijenbroek, M.; Nielsen, M.; Corver, W.E.; Jordanova, E.S.; ter Haar, N.; Tops, C.M.J.; Vasen, H.F.A.; Lips, E.H.; van Eijk, R.; et al. High frequency of copy-neutral LOH in MUTYH-associated polyposis carcinomas. J. Pathol. 2008, 216, 25–31. [Google Scholar] [CrossRef]
- Lubbe, S.J.; Di Bernardo, M.C.; Chandler, I.P.; Houlston, R.S. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J. Clin. Oncol. 2009, 27, 3975–3980. [Google Scholar] [CrossRef]
- Nielsen, M.; de Miranda, N.F.C.C.; van Puijenbroek, M.; Jordanova, E.S.; Middeldorp, A.; van Wezel, T.; van Eijk, R.; Tops, C.M.J.; Vasen, H.F.A.; Hes, F.J.; et al. Colorectal carcinomas in MUTYH-associated polyposis display histopathological similarities to microsatellite unstable carcinomas. BMC Cancer 2009, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, J.H.; Rodrigue, C.M.; Mourra, N.; Bennis, M.; Flejou, J.-F.; Parc, R.; Tiret, E.; Gespach, C.; Parc, Y.R. Implication of MYH in colorectal polyposis. Ann. Surg. 2006, 244, 874–879; discussion 879–880. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Freeman-Mills, L.; Rayner, E.; Glaire, M.; Briggs, S.; Vermeulen, L.; Fessler, E.; Medema, J.P.; Boot, A.; Morreau, H.; et al. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: A retrospective, pooled biomarker study. Lancet Gastroenterol. Hepatol. 2016, 1, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dong, L.; Liu, X.; Ou, K.; Yang, L. POLE/POLD1 mutation and tumor immunotherapy. J. Exp. Clin. Cancer Res. 2022, 41, 216. [Google Scholar] [CrossRef]
- Carethers, J.M.; Jung, B.H. Genetics and Genetic Biomarkers in Sporadic Colorectal Cancer. Gastroenterology 2015, 149, 1177–1190.e3. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Shimizu, E.; Yamaguchi, R.; Imoto, S.; Komura, M.; Hatakeyama, S.; Noguchi, R.; Takane, K.; Ilkenoue, T.; Gohda, Y.; et al. Development of an MSI-positive colon tumor with aberrant DNA methylation in a PPAP patient. J. Hum. Genet. 2019, 64, 729–740. [Google Scholar] [CrossRef]
- Chen, J.; Lou, H. Complete Response to Pembrolizumab in Advanced Colon Cancer Harboring Somatic POLE F367S Mutation with Microsatellite Stability Status: A Case Study. OncoTargets Ther. 2021, 14, 1791–1796. [Google Scholar] [CrossRef]
- Gong, J.; Wang, C.; Lee, P.P.; Chu, P.; Fakih, M. Response to PD-1 Blockade in Microsatellite Stable Metastatic Colorectal Cancer Harboring a POLE Mutation. J. Natl. Compr. Cancer Netw. 2017, 15, 142–147. [Google Scholar] [CrossRef]
- Garmezy, B.; Gheeya, J.; Lin, H.Y.; Huang, Y.; Kim, T.; Jiang, X.; Thein, K.Z.; Pilie, P.G.; Zeineddine, F.; Wang, W.; et al. Clinical and Molecular Characterization of POLE Mutations as Predictive Biomarkers of Response to Immune Checkpoint Inhibitors in Advanced Cancers. JCO Precis. Oncol. 2022, 6, e2100267. [Google Scholar] [CrossRef]
- Cunningham, J.M.; Christensen, E.R.; Tester, D.J.; Kim, C.Y.; Roche, P.C.; Burgart, L.J.; Thibodeau, S.N. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998, 58, 3455–3460. [Google Scholar]
- Parsons, M.T.; Buchanan, D.D.; Thompson, B.; Young, J.P.; Spurdle, A.B. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: A literature review assessing utility of tumour features for MMR variant classification. J. Med. Genet. 2012, 49, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Lièvre, A.; de la Fouchardière, C.; Samalin, E.; Benoist, S.; Phelip, J.M.; André, T.; Lledo, G. BRAF V600E-mutant colorectal cancers: Where are we? Bull. Cancer 2020, 107, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, P.; Kuchiba, A.; Imamura, Y.; Liao, X.; Yamauchi, M.; Nishihara, R.; Qian, Z.R.; Morikawa, T.; Shen, J.; Meyerhardt, J.A. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J. Natl. Cancer Inst. 2013, 105, 1151–1156. [Google Scholar] [CrossRef]
- Tran, B.; Kopetz, S.; Tie, J.; Gibbs, P.; Jiang, Z.-Q.; Lieu, C.H.; Agarwal, A.; Maru, D.M.; Sieber, O.; Desai, J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011, 117, 4623–4632. [Google Scholar] [CrossRef]
- Maloberti, T.; De Leo, A.; Sanza, V.; Merlo, L.; Visani, M.; Acquaviva, G.; Coluccelli, S.; Altimari, A.; Gruppioni, E.; Zagnoni, S.; et al. BRAF and MLH1 Analysis Algorithm for the Evaluation of Lynch Syndrome Risk in Colorectal Carcinoma Patients: Evidence-Based Data from the Analysis of 100 Consecutive Cases. J. Mol. Pathol. 2022, 3, 115–124. [Google Scholar] [CrossRef]
- Bläker, H.; Haupt, S.; Morak, M.; Holinski-Feder, E.; Arnold, A.; Horst, D.; Sieber-Frank, J.; Seidler, F.; von Winterfeld, M.; Alwers, E.; et al. Age-dependent performance of BRAF mutation testing in Lynch syndrome diagnostics. Int. J. Cancer 2020, 147, 2801–2810. [Google Scholar] [CrossRef]
- Young, J.; Simms, L.A.; Biden, K.G.; Wynter, C.; Whitehall, V.; Karamatic, R.; George, J.; Goldblatt, J.; Walpole, I.; Robin, S.A.; et al. Features of Colorectal Cancers with High-Level Microsatellite Instability Occurring in Familial and Sporadic Settings: Parallel Pathways of Tumorigenesis. Am. J. Path. 2001, 159, 2107–2116. [Google Scholar] [CrossRef]
- Jass, J.R.; Walsh, M.D.; Barker, M.; Simms, L.A.; Young, J.; Leggett, B.A. Distinction between familial and sporadic forms of colorectal cancer showing DNA microsatellite instability. Eur. J. Cancer 2002, 38, 858–866. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar]
- Lu, K.H.; Dinh, M.; Kohlmann, W.; Watson, P.; Green, J.; Syngal, S.; Bandipallium, P.; Chen, L.-M.; Allen, B.; Conrad, P.; et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet. Gynecol. 2005, 105, 569–574. [Google Scholar] [CrossRef]
- Walsh, C.S.; Hacker, K.E.; Secord, A.A.; DeLair, D.F.; McCourt, C.; Urban, R. Molecular testing for endometrial cancer: An SGO clinical practice statement. Gynecol. Oncol. 2023, 168, 48–55. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, and Gastric, in: NCCN Guidelines Version 3.2024; NCCN: Fort Washington, PA, USA, 2024; Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_ceg.pdf (accessed on 18 March 2025).
- American College of Obstetricians and Gynecologists. Committee opinion no. 634: Hereditary cancer syndromes and risk assessment. Obstet. Gynecol. 2015, 125, 1538–1543. [Google Scholar]
- Kurnit, K.C.; Westin, S.N.; Coleman, R.L. Microsatellite instability in endometrial cancer: New purpose for an old test. Cancer 2019, 125, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Jumaah, A.S.; Al-Haddad, H.S.; Salem, M.M.; McAllister, K.A.; Yasseen, A.A. Mismatch repair deficiency and clinicopathological characteristics in endometrial carcinoma: A systematic review and meta-analysis. J. Pathol. Transl. Med. 2021, 55, 202–211. [Google Scholar] [CrossRef]
- McMeekin, D.S.; Tritchler, D.L.; Cohn, D.E.; Mutch, D.G.; Lankes, H.A.; Geller, M.A.; Powell, M.A.; Backes, F.J.; Landrum, L.M.; Zaino, R.; et al. Clinicopathologic Significance of Mismatch Repair Defects in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2016, 34, 3062–3068. [Google Scholar] [CrossRef]
- Cosgrove, C.M.; Cohn, D.E.; Hampel, H.; Frankel, W.L.; Jones, D.; McElroy, J.P.; Suarez, A.A.; Zhao, W.; Chen, W.; Salani, R.; et al. Epigenetic silencing of MLH1 in endometrial cancers is associated with larger tumor volume, increased rate of lymph node positivity and reduced recurrence-free survival. Gynecol. Oncol. 2017, 146, 588–595. [Google Scholar] [CrossRef]
- Ruiz, I.; Martin-Arruti, M.; Lopez-Lopez, E.; Garcia-Orad, A. Lack of association between deficient mismatch repair expression and outcome in endometrial carcinomas of the endometrioid type. Gynecol. Oncol. 2014, 134, 20–23. [Google Scholar] [CrossRef]
- Manning-Geist, B.L.; Lui, Y.L.; Devereaux, K.A.; Paula, A.D.C.; Zhou, Q.C.; Ma, W.; Selenica, P.; Ceyhan-Birsoy, O.; Moukarzel, L.A.; Hoang, T.; et al. Microsatellite Instability–High Endometrial Cancers with MLH1 Promoter Hypermethylation Have Distinct Molecular and Clinical Profiles. Clin. Cancer Res. 2022, 28, 4302–4311. [Google Scholar] [CrossRef]
- Pasanen, A.; Loukovaara, M.; Butzow, R. Clinicopathological significance of deficient DNA mismatch repair and MLH1 promoter methylation in endometrioid endometrial carcinoma. Mod. Pathol. 2020, 33, 1443–1452. [Google Scholar] [CrossRef]
- Post, C.C.B.; Stelloo, E.; Smit, V.T.H.B.M.; Ruano, D.; Tops, C.M.; Vermij, L.; Rutten, T.A.; Jurgenliemk-Schulz, I.M.; Lutgens, L.C.H.W.; Jobsen, J.J.; et al. Prevalence and prognosis of lynch syndrome and sporadic mismatch repair deficiency in endometrial cancer. J. Natl. Cancer Inst. 2021, 113, 1212–1220. [Google Scholar] [CrossRef]
- Velho, S.; Fernandes, M.S.; Leite, M.; Figueiredo, C.; Seruca, R. Causes and consequences of microsatellite instability in gastric carcinogenesis. World J. Gastroenterol. 2014, 20, 16433–16442. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Beghelli, S.; de Manzoni, G.; Barbi, S.; Tomezzoli, A.; Roviello, F.; Di Gregorio, C.; Vindigni, C.; Bortesi, L.; Parisi, A.; Saragoni, L.; et al. Microsatellite instability in gastric cancer is associated with better prognosis in only stage II cancers. Surgery 2006, 139, 347–356. [Google Scholar] [CrossRef]
- Lee, H.S.; Choi, S.I.; Lee, H.K.; Kim, H.S.; Yang, H.-K.; Kang, G.H.; Kim, Y.I.; Lee, B.L.; Kim, W.H. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod. Pathol. 2002, 15, 632–640. [Google Scholar] [CrossRef]
- Seo, H.M.; Chang, Y.S.; Joo, S.H.; Kim, Y.W.; Park, Y.-K.; Hong, S.W.; Lee, S.-H. Clinicopathologic characteristics and outcomes of gastric cancers with the MSI-H phenotype. J. Surg. Oncol. 2008, 99, 143–147. [Google Scholar] [CrossRef]
- Gutierrez, C.; Ogino, S.; Meyerhardt, J.A.; Iorgulescu, J.B. The prevalence and prognosis of microsatellite instability-high/mismatch repair-deficient colorectal adenocarcinomas in the United States. JCO Precis. Oncol. 2023, 7, e2200179. [Google Scholar] [CrossRef]
- Kim, H.S.; Woo, D.K.; Bae, S.I.; Kim, Y.I.; Kim, W.H. Microsatellite instability in the adenoma-carcinoma sequence of the stomach. Lab. Investig. 2000, 80, 57–64. [Google Scholar] [CrossRef]
- Leung, S.Y.; Yuen, S.T.; Chung, L.P.; Chu, K.M.; Chan, A.S.; Ho, J.C. hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res. 1999, 59, 159–164. [Google Scholar]
- Fleisher, A.S.; Esteller, M.; Wang, S.; Tamura, G.; Suzuki, H.; Yin, J.; Zou, T.T.; Abraham, J.M.; Kong, D.; Smolinski, K.N.; et al. Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res. 1999, 59, 1090–1095. [Google Scholar]
- Thibodeau, S.N.; French, A.J.; Roche, P.C.; Cunningham, J.M.; Tester, D.J.; Lindor, N.M.; Moslein, G.; Baker, S.M.; Liskay, R.M.; Burgart, L.J.; et al. Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res. 1996, 56, 4836–4840. [Google Scholar]
- Halling, K.C.; Harper, J.; Moskaluk, C.A.; Thibodeau, S.N.; Petroni, G.R.; Yustein, A.S.; Tosi, P.; Minacci, C.; Roviello, F.; Piva, P.; et al. Origin of Microsatellite Instability in Gastric Cancer. Am. J. Pathol. 1999, 155, 205–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chandran, E.B.; Iannantuono, G.M.; Atiq, S.O.; Akbulut, D.; Sinaii, N.; Simon, N.I.; Banday, A.R.; Boudjadi, S.; Gurram, S.; Nassar, A.H.; et al. Mismatch repair deficiency and microsatellite instability in urothelial carcinoma: A systematic review and meta-analysis. BMJ Oncol. 2024, 3, e000335. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.; Loeffler, M.; Steinke, V.; Rahner, N.; Holinski-Feder, E.; Dietmaier, W.; Shackert, H.K.; Goergens, H.; von Knebel Doeberitz, M.; Goecke, T.O.; et al. Risks of less common cancers in proven mutation carriers with lynch syndrome. J. Clin. Oncol. 2012, 30, 4409–4415. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Seppälä, T.T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.H. Cancer risk and survival in Path Mmr carriers by gene and gender up to 75 years of age: A report from the prospective lynch syndrome database. Gut 2018, 67, 1306–1316. [Google Scholar] [CrossRef]
- Bhalla, A.; Zulfiqar, M.; Weindel, M.; Shidham, V.B. Molecular diagnostics in colorectal carcinoma. Clin. Lab. Med. 2013, 33, 835–859. [Google Scholar] [CrossRef]
- Hartmann, A.; Cheville, J.C.; Dietmaier, W.; Hofstadter, F.; Burgart, L.J.; Blaszyk, H. Hereditary nonpolyposis colorectal cancer syndrome in a patient with urothelial carcinoma of the upper urothelial tract. Arch. Pathol. Lab. Med. 2003, 127, E60–E63. [Google Scholar] [CrossRef]
- Gibson, J.; Lacy, J.; Matloff, E.; Robert, M. Microsatellite instability testing in colorectal carcinoma: A practical guide. Clin. Gastroenterol. Hepatol. 2014, 12, 171–176. [Google Scholar] [CrossRef]
- Harper, H.; McKenney, J.; Heald, B.; Stephenson, A.; Campbell, S.C.; Plesec, T.; Magi-Galluzzi, C. Upper tract urothelial carcinomas: Frequency of association with mismatch repair protein loss and lynch syndrome. Mod. Pathol. 2017, 30, 146–156. [Google Scholar] [CrossRef]
- Therkildsen, C.; Eriksson, P.; Höglund, M.; Jonsson, M.; Sjodahl, G.; Nilbert, M.; Liedberg, F. Molecular subtype classification of urothelial carcinoma in lynch syndrome. Mol. Oncol. 2018, 12, 1286–1295. [Google Scholar] [CrossRef]
- Rao, A.; McGrath, J.E.; Xiu, J.; De Souza, A.; Gulati, S.; Abuali, I.; Sagaram, S.; Nabhan, C.; Korn, W.M.; Ryan, C.; et al. Characterization of microsatellite instability (dMMR/MSI-H) and mutational landscape in a large contemporary cohort of upper tract urothelial cancer (UTUC) patients. J. Clin. Oncol. 2021, 39, 465. [Google Scholar] [CrossRef]
- Mylona, E.; Zarogiannos, A.; Nomikos, A.; Giannopoulo, I.; Nikolaou, I.; Zervas, A.; Nakopoulou, L. Prognostic value of microsatellite instability determined by immuno-histochemical staining of hMSH2 and hMSH6 in urothelial carcinoma of the bladder. APMIS 2008, 116, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Fromont, G.; Azzouzi, A.-R.; Catto, J.W.; Vallancien, G.; Hamdy, F.C.; Cussenot, O. Microsatellite instability as predictor of survival in patients with invasive upper urinary tract transitional cell carcinoma. Urology 2005, 65, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Saetta, A.A.; Goudopoulou, A.; Korkolopoulou, P.; Voutsinas, G.; Thomas-Tsagli, E.; Michalopoulous, N.V.; Patsouris, E. Mononucleotide markers of microsatellite instability in carcinomas of the urinary bladder. Eur. J. Surg. Oncol. 2004, 30, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Tural, D.; Akar, E.; Baytekin, H.F.; Canoglu, D.; Yilmaz, M.; Tugcu, V. Relationship between survival outcomes and microsatellite instability, tumor infiltrating lymphocytes and programmed cell death Ligand-1 expression in patients with bladder cancer and radical cystectomy. J. Buon 2021, 26, 2117–2125. [Google Scholar]
- Hu, Z.I.; Shia, J.; Stadler, Z.K.; Varghese, A.M.; Capanu, M.; Salo-Mullen, E.; Lowery, M.A.; Diaz, L.A., Jr.; Mandelker, D.; Yu, K.H.; et al. Evaluating Mismatch Repair Deficiency in Pancreatic Adenocarcinoma: Challenges and Recommendations. Clin. Cancer Res. 2018, 24, 1326–1336. [Google Scholar] [CrossRef]
- Liu, W.; Shia, J.; Gonen, M.; Lowery, M.A.; O’Reilly, E.M.; Klimstra, D.S. DNA mismatch repair abnormalities in acinar cell carcinoma of the pancreas: Frequency and clinical significance. Pancreas 2014, 43, 1264–1270. [Google Scholar] [CrossRef]
- Yamamoto, H.; Itoh, F.; Nakamura, H.; Fukushima, H.; Sasaki, S.; Perucho, M.; Imai, K. Genetic and clinical features of human pancreatic ductal adenocarcinomas with widespread microsatellite instability. Cancer Res. 2001, 61, 31393144. [Google Scholar]
- Nakata, B.; Wang, Y.Q.; Yashiro, M.; Nishioka, N.; Tanaka, H.; Ohira, M.; Ishiwaka, T.; Nishino, H.; Hirakawa, K. Prognostic value of microsatellite instability in resectable pancreatic cancer. Clin. Cancer Res. 2002, 8, 2536–2540. [Google Scholar]
- Lee, V.; Murphy, A.; Le, D.T.; Diaz, L.A., Jr. Mismatch repair deficiency and response to immune checkpoint blockade. Oncologist 2016, 21, 1200–1211. [Google Scholar] [CrossRef]
- Vivaldi, C.; Genovesi, V.; Ugolini, C.; Bernardini, L.; Casadei-Gardini, A.; Formica, V.; Salani, F.; Orsi, G.; Massa, V.; Cacciato-Insilla, A.; et al. Mismatch Repair Deficiency in Biliary Tract Cancer: Prognostic Implications and Correlation with Histology. Oncology 2024, 102, 157–167. [Google Scholar] [CrossRef]
- Yang, X.; Lian, B.; Zhang, N.; Long, J.; Li, Y.; Xue, J.; Chen, X.; Wang, Y.; Xun, Z.; Piao, M.; et al. Genomic characterization and immunotherapy for microsatellite instability-high in cholangiocarcinoma. BMC Med. 2024, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Christodoulidis, G.; Kouliou, M.N.; Koumarelas, K.E. Immune signature of small bowel adenocarcinoma and the role of tumor microenvironment. World J. Gastroenterol. 2024, 30, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Planck, M.; Ericson, K.; Piotrowska, Z.; Halvarsson, B.; Rambech, E.; Nilbert, M. Microsatellite Instability and Expression of MLH1 and MSH2 in Carcinomas of the Small Intestine. Cancer 2003, 97, 1551–1557. [Google Scholar] [CrossRef]
- Wirta, E.-V.; Szeto, S.; Hänninen, U.; Ahtiainen, M.; Bohm, J.; Mecklin, J.-P.; Aaltonen, L.A.; Seppala, T.T. Prognostic Value of Immune Environment Analysis in Small Bowel Adenocarcinomas with Verified Mutational Landscape and Predisposing Conditions. Cancers 2020, 12, 2018. [Google Scholar] [CrossRef]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Lenis, A.T.; Ravichandran, V.; Truong, H.; Reisz, P.; Nweji, B.; Autio, K.; Morris, M.; Slovin, S.; Vargas, H.A.; Laudone, V.; et al. Response to immune checkpoint blockade in patients with microsatellite instable and high tumor mutational burden prostate cancer. In Proceedings of the 2021 American Urological Association Annual Meeting, Virtual, 10–13 September 2021. Abstract MP24-01. [Google Scholar]
- Hu, Q.; Rizvi, A.A.; Schau, G.; Ingale, K.; Muller, Y.; Baits, R.; Pretzer, S.; BenTaieb, A.; Gordhamer, A.; Nussenzveig, R.; et al. Development and validation of a deep learning-based microsatellite instability predictor from prostate cancer whole-slide images. NPJ Precis. Oncol. 2024, 8, 88. [Google Scholar] [CrossRef]
- Pal, T.; Permuth-Wey, J.; Kumar, A.; Sellers, T.A. Systematic review and meta-analysis of ovarian cancers: Estimation of microsatellite-high frequency and characterization of mismatch repair deficient tumor histology. Clin. Cancer Res. 2008, 14, 6847–6854. [Google Scholar] [CrossRef]
- Fraune, C.; Rosebrock, J.; Simon, R.; Hube-Magg, C.; Makrypidi-Fraune, G.; Kluth, M.; Buscheck, F.; Hoflmayer, D.; Schmalfeldt, B.; Muller, V. High homogeneity of MMR deficiency in ovarian cancer. Gynecol. Oncol. 2020, 156, 669–675. [Google Scholar] [CrossRef]
- Raymond, V.M.; Everett, J.N.; Furtado, L.V.; Gustafson, S.L.; Jungbluth, C.R.; Gruber, S.B.; Hammer, G.D.; Stoffel, E.M.; Greenson, J.K.; Giordano, T.J. Adrenocortical carcinoma is a Lynch syndrome-associated cancer. J. Clin. Oncol. 2013, 31, 3012–3018. [Google Scholar] [CrossRef]
- Rocha, L.M.; Schmid, W.K.; Czapiewski, P. The prevalence of DNA microsatellite instability in anaplastic thyroid carcinoma—Systematic review and discussion of current therapeutic options. Contemp. Oncol. 2021, 25, 213–223. [Google Scholar]
- Tepeoglu, M.; Borcek, P.; Ozen, O.; Altinors, N. Microsatellite instability in glioblastoma: Is it really relevant in tumor prognosis? Turk. Neurosurg. 2019, 29, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Touat, M.; Li, Y.Y.; Boynton, A.N.; Spurr, L.F.; Iorgulescu, J.B.; Bohrson, C.L.; Cortes-Ciriano, I.; Birzu, C.; Geduldig, J.E.; Pelton, K. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 2020, 580, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Cani, M.; Novello, S.; Bironzo, P. Mismatch repair deficiency in lung tumors: Adding a new layer of complexity on pie slices. J. Thorac. Oncol. 2024, 19, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wang, H.; Lu, C.; Liu, L.; Zhang, X.; Xie, Y.; Li, R.; Lv, X.; Fu, D.; Zhang, L. Genomic characteristics and prognosis of lung cancer patients with MSI-H: A cohort study. Lung Cancer 2023, 181, 107255. [Google Scholar] [CrossRef]
- Qin, J.; Shi, D.; Yin, Y.; Liu, B.; Wang, L.; Sun, T.; Zhang, Q.; Qi, C. The clinical and genomic characteristics of MSI-H/dMMR lung cancer. J. Clin. Oncol. 2022, 40 (Suppl. S16), e21142. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Nathanson, T.; Rizvi, H.; Creelan, B.C.; Sanchez-Vega, F.; Ahuja, A.; Ni, A.; Novik, J.B.; Mangarin, L.M.B.; Abu-Akeel, M.; et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell 2018, 33, 843–852.e4. [Google Scholar] [CrossRef]
- Illergard, K.; Soto, P.; Arpegård, J.; Schiza, A.; Pahnke, S. Immune checkpoint inhibitor treatment—Real-world overall survival, treatment adoption rates, and regional differences in Sweden. SSRN Electron. J. 2024, ssrn.5128524. [Google Scholar]
- Schrock, A.B.; Ouyang, C.; Sandhu, J.; Sokol, E.; Jin, D.; Ross, J.S.; Miller, V.A.; Lim, D.; Amanam, I.; Chao, J.; et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann. Oncol. 2019, 30, 1096–1103. [Google Scholar] [CrossRef]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.G.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumor mutational burden with outcomes in patients with select advanced solid tumors treated with pembrolizumab in KEYNOTE-158. Ann. Oncol. 2019, 30 (Suppl. S5), v475–v532. [Google Scholar] [CrossRef]
- Ozer, M.; Vegivinti, C.T.R.; Syed, M.; Ferrell, M.E.; Gonzalez Gomez, C.; Cheng, S.; Holder-Murray, J.; Bruno, T.; Saeed, A.; Sahin, I.H. Neoadjuvant Immunotherapy for Patients with dMMR/MSI-High Gastrointestinal Cancers: A Changing Paradigm. Cancers 2023, 15, 3833. [Google Scholar] [CrossRef] [PubMed]
- Naulaerts, S.; Datsi, A.; Borras, D.M.; Antoranz Martinez, A.; Messiaen, J.; Vanmeerbeek, I.; Sprooten, J.; Laureano, R.S.; Govaerts, J.; Panovska, D.; et al. Multiomics and spatial mapping characterizes human CD8+ T cell states in cancer. Sci. Transl. Med. 2023, 15, eadd1016. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Hu, H.; Qin, G.; Wu, X.; Bai, F.; Zhang, J.; Cai, Y.; Huang, Y.; Wang, C.; et al. Remodeling of the immune and stromal cell compartment by PD-1 blockade in mismatch repair-deficient colorectal cancer. Cancer Cell 2023, 41, 1152–1169.e7. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Cercek, A.; Sinopoli, J.C.; Shia, J.; Weiss, J.; Temple, L.; Smith, J.J.; Saltz, L.B.; Widmar, M.; Fumo, G.; Aparo, S.; et al. Durable complete responses to PD-1 blockade alone in mismatch repair deficient locally advanced rectal cancer. J. Clin. Oncol. 2024, 42, LBA3512. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef]
- Chalabi, M.; Verschoor, Y.L.; Tan, P.B.; Balduzzi, S.; Van Lent, A.U.; Grootscholten, C.; Dokter, S.; Büller, N.V.; Grotenhuis, B.A.; Kuhlmann, K. Neoadjuvant Immunotherapy in Locally Advanced Mismatch Repair-Deficient Colon Cancer. N. Engl. J. Med. 2024, 390, 949–1958. [Google Scholar] [CrossRef]
- Shiu, K.K.; Jiang, Y.; Saunders, M.; Seligmann, J.F.; Iveson, T.; Wilson, R.H.; Graham, J.S.; Khan, K.H.; Militello, A.-M.; Irvine, S.; et al. NEOPRISM-CRC: Neoadjuvant pembrolizumab stratified to tumour mutation burden for high risk stage 2 or stage 3 deficient-MMR/MSI-high colorectal cancer. J. Clin. Oncol. 2024, 42 (Suppl. S17), LBA3504. [Google Scholar] [CrossRef]
- Neoadjuvant Pembrolizumab May Improve Outcomes in Some Patients with Colorectal Cancer Surgery. Available online: https://ascopost.com/news/june-2024/neoadjuvant-pembrolizumab-may-improve-outcomes-in-some-patients-with-colorectal-cancer-surgery (accessed on 20 March 2025).
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lievre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Lorenzen, S.; Homann, N.; Thuss-Patience, P.C.; Schenk, M.; Lindig, U.; Kretzschmar, A.; Huere, V.; Goekkurt, E.; Haag, G.M.; et al. 1429P Pathological regression in patients with microsatellite instability (MSI) receiving perioperative atezolizumab in combination with FLOT vs. FLOT alone for resectable esophagogastric adenocarcinoma: Results from the DANTE trial of the German Gastric Group at the AIO and SAKK. Ann. Oncol. 2021, 32, S1069. [Google Scholar]
- Thierry, A.; Tougeron, D.; Piessen, G.; de la Fouchardiere, C.; Louvet, C.; Adenis, A.; Jary, M.; Tournigand, C.; Aparicio, T.; Desrame, J.; et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability–High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J. Clin. Oncol. 2023, 41, 255–265. [Google Scholar]
- Coutzac, C.; Bibeau, F.; Abdelghani, M.B.; Aparicio, T.; Cohen, R.; Coquan, E.; Dubreuil, O.; Evesque, L.; Ghiringhelli, F.; Kim, S.; et al. Immunotherapy in MSI/dMMR tumors in the perioperative setting: The IMHOTEP trial. Dig. Liver Dis. 2022, 54, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Lipson, E.J.; Sharfman, W.H.; Drake, C.G.; Wollner, I.; Taube, J.M.; Anders, R.A.; Xu, H.; Yao, S.; Pons, A.; Chen, L.; et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin. Cancer Res. 2013, 19, 462–468. [Google Scholar] [CrossRef]
- FDA Approves First Cancer Treatment for Any Solid Tumor with a Specific Genetic Feature. Available online: https://www.drugs.com/newdrugs/fda-approves-keytruda-pembrolizumab-first-cancer-any-solid-tumor-specific-ge-netic-feature-4538.html (accessed on 18 March 2025).
- FDA Approves Merck’s KEYTRUDA® (Pembrolizumab) for Adult and Pediatric Patients with Unresectable or Metastatic, Microsatellite Instability-High (MSI-H) or Mismatch Repair Deficient (dMMR) Solid Tumors. Available online: https://www.merck.com/news/fda-approves-mercks-keytruda-pembrolizumab-for-adult-and-pediatric-patients-with-unresectable-or-metastatic-microsatellite-instability-high-msi-h-or-mismatch-repair-deficient-dmmr/#:~:text=This%20accelerated%20FDA%20approval%20for,Johns%20Hopkins%20Bloomberg%2DKim-mel%20Institute (accessed on 19 March 2025).
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191, Erratum in Lancet Oncol. 2017, 18, 510. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, M.; Ghidini, A.; Tomasello, G. Outcomes Following Immune Checkpoint Inhibitor Treatment of Patients with Microsatellite Instability-High Cancers: A Systematic Review and Meta-analysis. JAMA Oncol. 2020, 6, 1068–1071. [Google Scholar] [CrossRef]
- Kluger, H.M.; Tawbi, H.A.; Ascierto, M.L.; Bowden, M.; Callahan, M.K.; Cha, E.; Chen, H.X.; Drake, C.G.; Feltquate, D.M.; Ferris, R.L.; et al. Defining tumor resistance to PD-1 pathway blockade: Recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce. J. Immunother. Cancer 2020, 8, e000398. [Google Scholar] [CrossRef]
- Ursino, C.; Mouric, C.; Gros, L.; Bonnefoy, N.; Faget, J. Intrinsic features of the cancer cell as drivers of immune checkpoint blockade response and refractoriness. Front. Immunol. 2023, 14, 1170321. [Google Scholar] [CrossRef]
- Pitt, J.M.; Vétizou, M.; Daillère, R.; Roberti, M.P.; Yamazaki, T.; Routy, B.; Lepage, P.; Boneca, I.G.; Chamaillard, M.; Kroemer, G.; et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity 2016, 44, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Ribic, C.M.; Sargent, D.J.; Moore, M.M.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent-Puig, P.; Gryfe, R.; Sheperd, L.E.; et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2003, 349, 247–257. [Google Scholar] [CrossRef]
- Sargent, D.J.; Marsoni, S.; Monges, G.; Thibodeau, S.N.; Labianca, R.; Hamilton, S.R.; French, A.J.; Kabat, B.; Foster, N.R.; Torri, V.; et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J. Clin. Oncol. 2010, 28, 3219–3226. [Google Scholar] [CrossRef]
- Cohen, R.; Taieb, J.; Fiskum, J.; Yothers, G.; Goldberg, R.; Yoshino, T.; Alberts, S.; Allegra, C.; de Gramont, A.; Seitz, J.F.; et al. Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: An ACCENT pooled analysis of 12 adjuvant trials. J. Clin. Oncol. 2021, 39, 642–651. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Miceli, R.; Raimondi, A.; Kim, Y.W.; Kang, W.K.; Langley, R.E.; Choi, Y.Y.; Kim, K.M.; Nankivell, M.G.; Morano, F. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability as a Biomarker in Gastric Cancer. J. Clin. Oncol. 2019, 37, 3392–3400. [Google Scholar] [CrossRef]
- FDA Approves Durvalumab with Chemotherapy for Mismatch Repair Deficient Primary Advanced or Recurrent Endometrial Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-chemotherapy-mismatch-repair-deficient-primary-advanced-or-recurrent (accessed on 14 June 2024).
| Hereditary MSI-H CRC | Sporadic MSI-H CRC | |
|---|---|---|
| Clinical Features |
|
|
| Incidence |
|
|
| Histopathology |
|
|
| Genomics |
|
|
| Microsatellite Status |
|
|
| Immunohistochemistry |
|
|
| Incidence/Clinical Features | Histopathology | Molecular Features | Prognosis | |
|---|---|---|---|---|
| Pancreatic ductal cancer |
|
|
|
|
| Biliary tract cancer |
|
|
| |
| Small bowel adenocarcinoma |
|
|
|
|
| Prostate adenocarcinoma |
|
|
| |
| Ovarian cancer |
|
|
| |
| Adrenocortical carcinoma |
|
|
| |
| Thyroid cancer |
|
|
|
|
| Glioblastoma |
|
|
| |
| Non-small-cell lung cancer |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awosika, J.A.; Gulley, J.L.; Pastor, D.M. Deficient Mismatch Repair and Microsatellite Instability in Solid Tumors. Int. J. Mol. Sci. 2025, 26, 4394. https://doi.org/10.3390/ijms26094394
Awosika JA, Gulley JL, Pastor DM. Deficient Mismatch Repair and Microsatellite Instability in Solid Tumors. International Journal of Molecular Sciences. 2025; 26(9):4394. https://doi.org/10.3390/ijms26094394
Chicago/Turabian StyleAwosika, Joy A., James L. Gulley, and Danielle M. Pastor. 2025. "Deficient Mismatch Repair and Microsatellite Instability in Solid Tumors" International Journal of Molecular Sciences 26, no. 9: 4394. https://doi.org/10.3390/ijms26094394
APA StyleAwosika, J. A., Gulley, J. L., & Pastor, D. M. (2025). Deficient Mismatch Repair and Microsatellite Instability in Solid Tumors. International Journal of Molecular Sciences, 26(9), 4394. https://doi.org/10.3390/ijms26094394






