Assessment of Catalase Inhibition Under e-Beam Irradiation
Abstract
1. Introduction
2. Results and Discussion
2.1. Research Stages
2.2. Catalase Activity in e-Beam Irradiated Solutions β at Different Doses
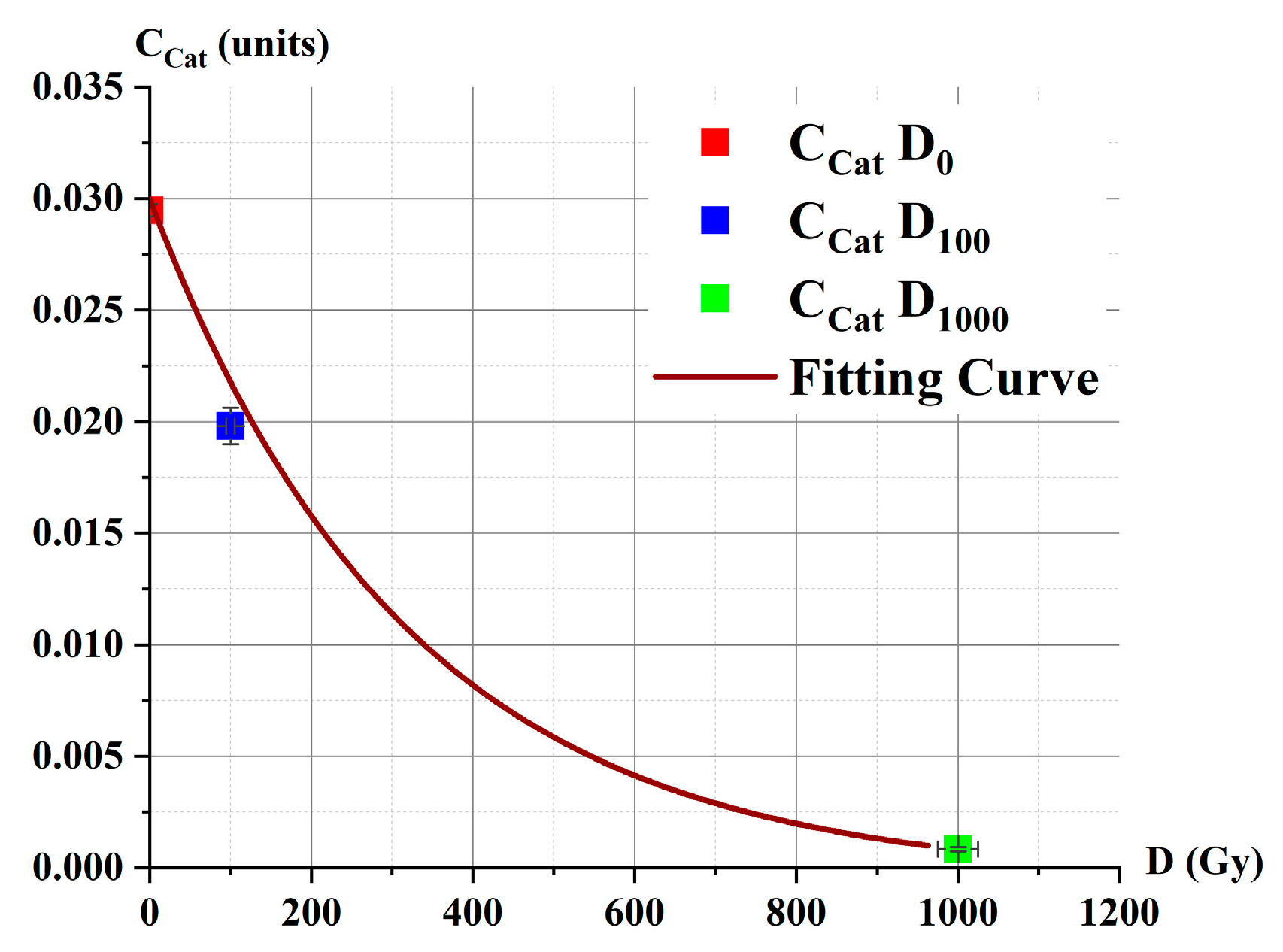
2.2.1. Catalase Activity in Irradiated Solutions β: Spectrophotometry Method
2.2.2. Catalase Activity in Irradiated Solutions β: Oxygen Bubble Method
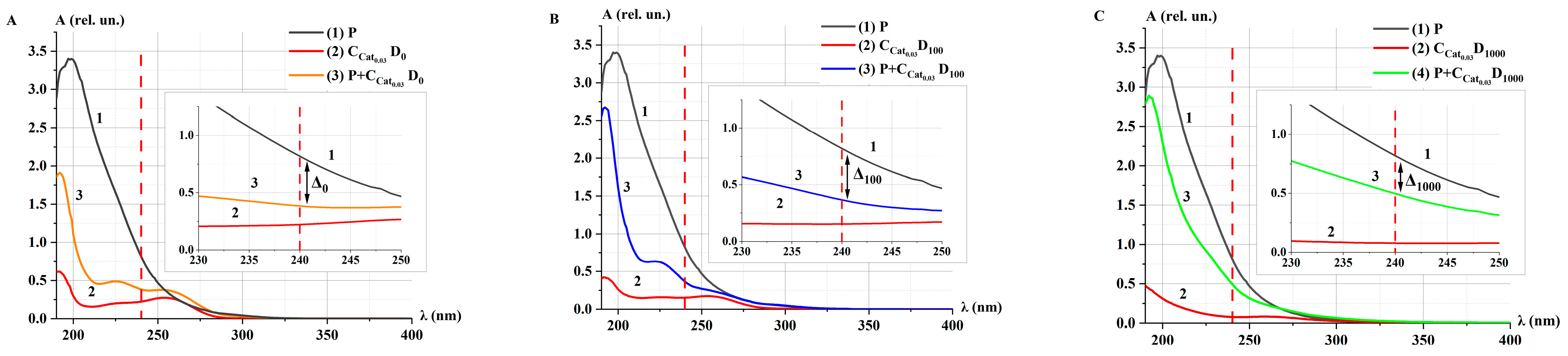
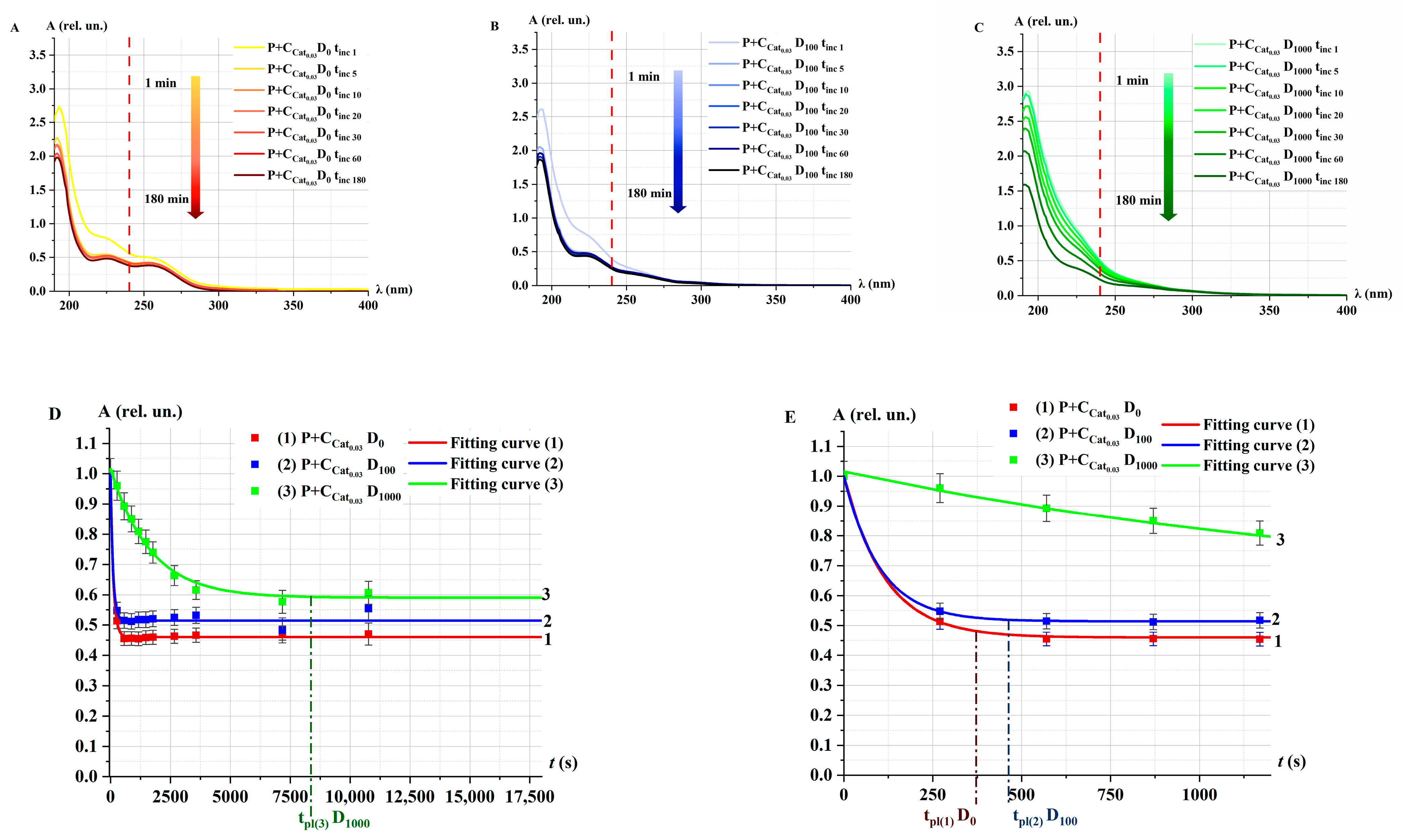
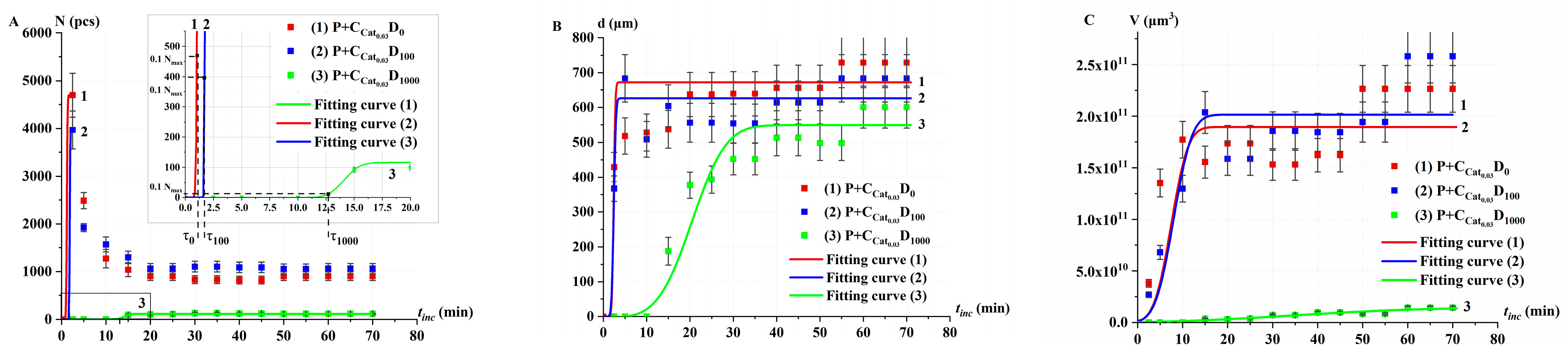
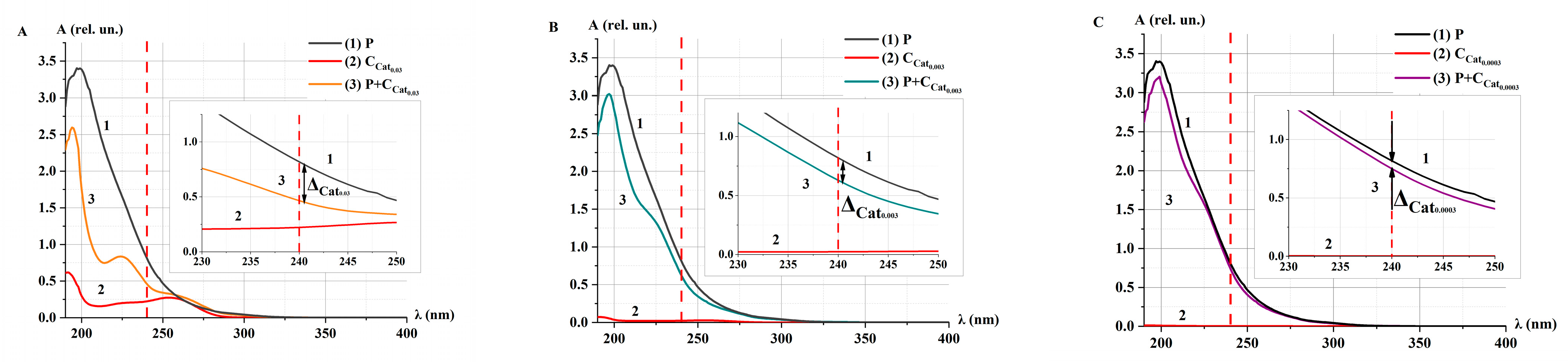
2.3. Catalase Activity in Non-Irradiated (Control) Solutions α at Different Concentrations
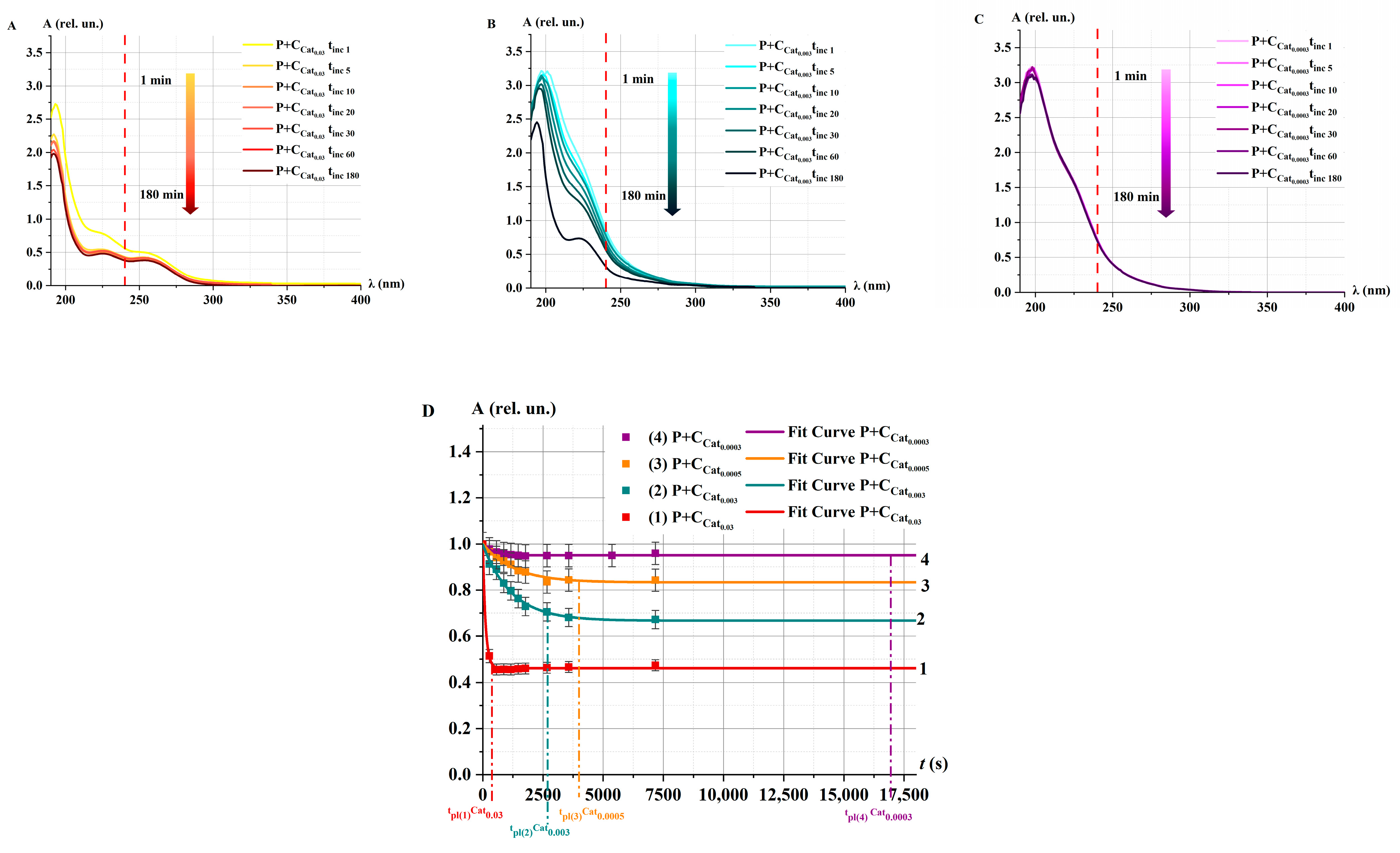
2.3.1. Catalase Activity in Non-Irradiated Solutions α: Spectrophotometry Method
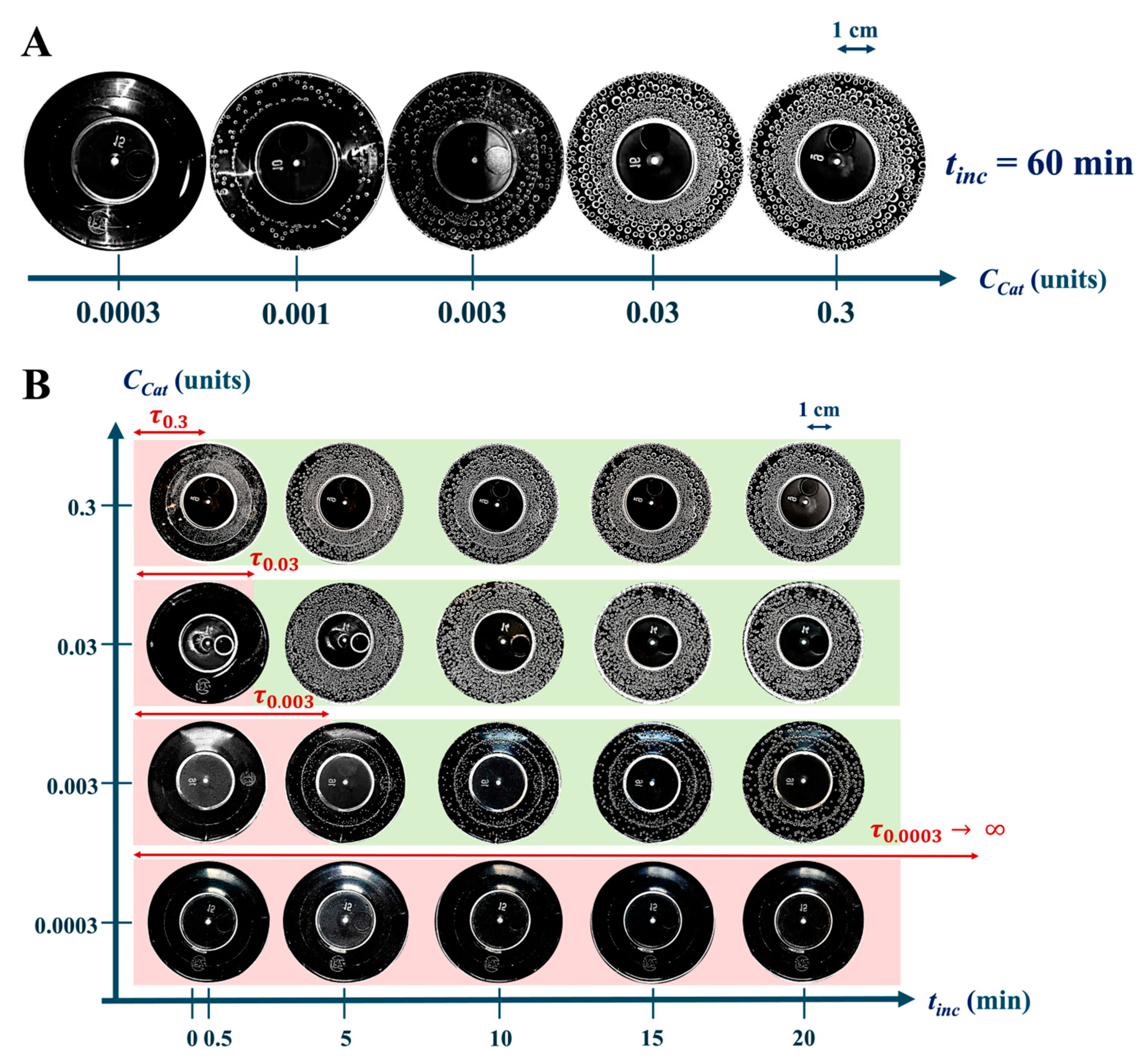
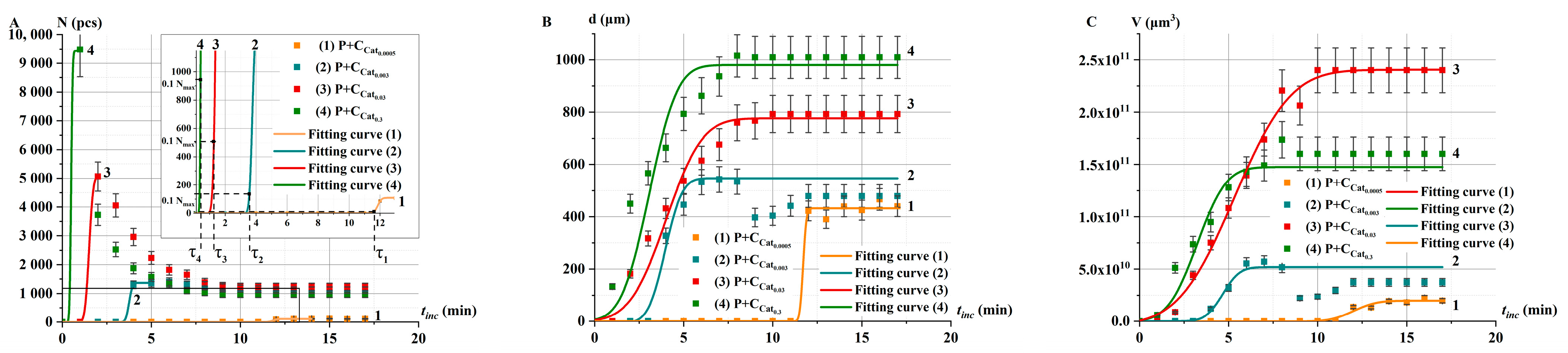
2.3.2. Catalase Activity in Non-Irradiated Solutions α: Oxygen Bubble Method
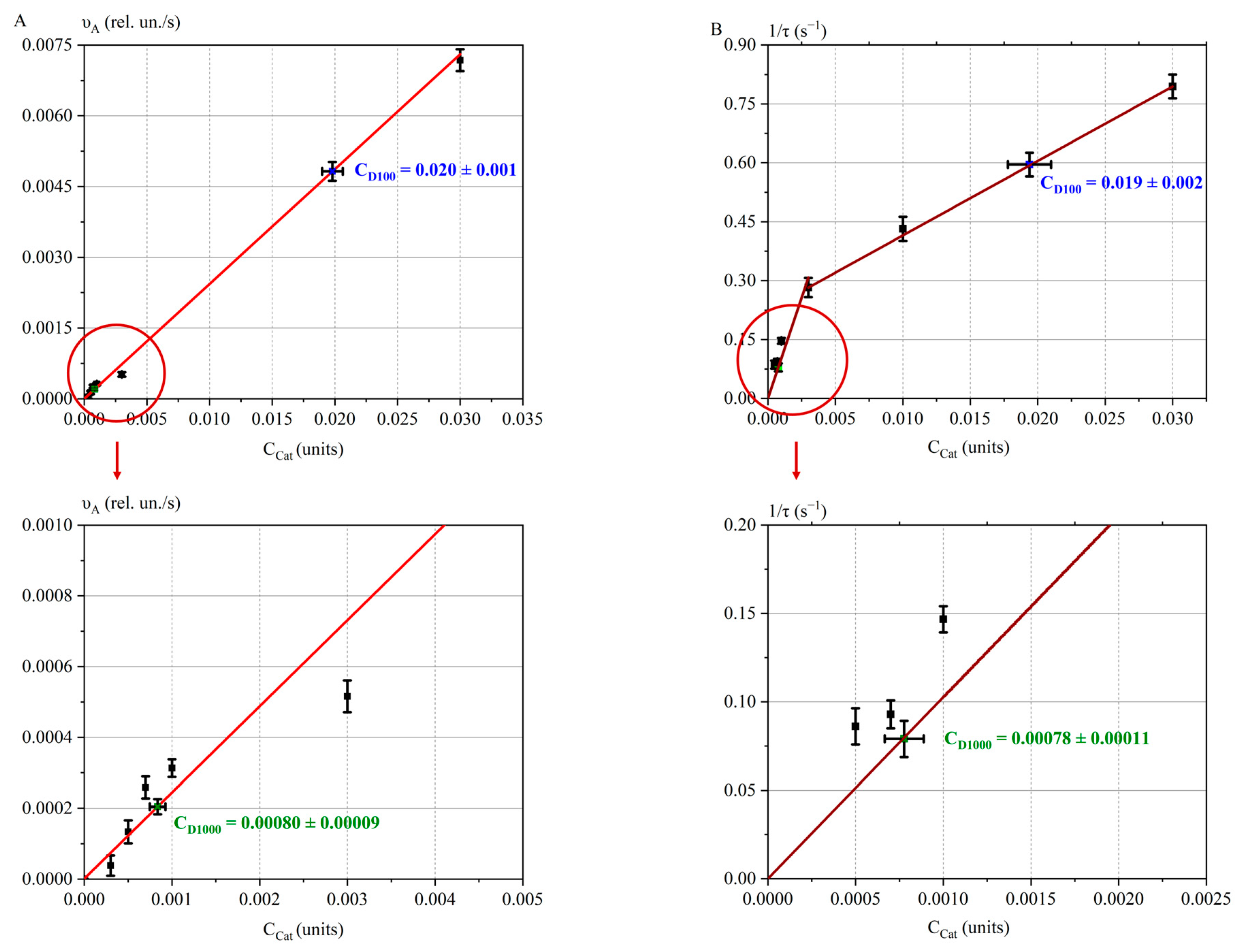
2.3.3. Calibration Curves for Doses
2.3.4. Catalase-Based Biodosimeter for Measuring High Irradiation Doses
3. Materials and Methods
3.1. Objects of Study and Sample Preparation
3.2. Experiment with Different Concentrations of Catalase
3.3. e-Beam Irradiation
3.4. Spectrophotometry Measurement
3.5. Oxygen Bubble Measurement
3.6. Construction of Calibration Curves
3.7. Statistical Analysis of the Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of Food Security and Nutrition in the World; FAO: Rome, Italy; IFAD: Rome, Italy; UNICEF: Rome, Italy; WFP: Rome, Italy; WHO: Rome, Italy, 2023; 316p. [Google Scholar] [CrossRef]
- IAEA. Manual of Good Practice in Food Irradiation; Technical Reports Series No. 481; IAEA: Vienna, Austria, 2015; pp. 1–104. [Google Scholar]
- Maherani, B.; Hossain, F.; Criado, P.; Ben-Fadhel, Y.; Salmieri, S.; Lacroix, M. World Market Development and Consumer Acceptance of Irradiation Technology. Foods 2016, 5, 79. [Google Scholar] [CrossRef]
- Commission, J.F.C.A. Codex General Standard for Irradiated Foods and Recommended International Code of Practice for the Operation of Radiation Facilities Used for the Treatment of Foods; Joint FAO/WHO Codex Alimentarius Commission: Rome, Italy, 1990. [Google Scholar]
- IAEA. Revision of the Recommended International General Standard for Irradiated Foods and of the Recommended International Code of Practice for the Operation of Radiation Facilities Used for the Treatment of Foods; Food and Agriculture Organization of the United Nations: Rome, Italy, 1981. [Google Scholar]
- World Health Organization. Food Irradiation: A Technique for Preserving and Improving the Safety of Food; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Innovating Radiation Processing of Food with Low Energy Beams from Machine Sources | IAEA. Available online: https://www.iaea.org/projects/crp/d61025 (accessed on 9 March 2025).
- Kozlova, E.; Bliznyuk, U.; Chernyaev, A.; Borshchegovskaya, P.; Braun, A.; Ipatova, V.; Zolotov, S.; Nikitchenko, A.; Chulikova, N.; Malyuga, A.; et al. Optimization Function for Determining Optimal Dose Range for Beef and Seed Potato Irradiation. Foods 2024, 13, 3729. [Google Scholar] [CrossRef] [PubMed]
- Erkmen, O.; Bozoglu, T.F. Food Microbiology: Principles into Practice; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; Volume 1, pp. 1–458. [Google Scholar] [CrossRef]
- ISO 14470:2011; Food Irradiation—Requirements for the Development, Validation and Routine Control of the Process of Irradiation Using Ionizing Radiation for the Treatment of Food. ISO: Geneva, Switzerland, 2011. Available online: https://www.iso.org/standard/44074.html (accessed on 9 March 2025).
- Danyo, E.K.; Ivantsova, M.N.; Selezneva, I.S. Ionizing Radiation Effects on Microorganisms and Its Applications in the Food Industry. Foods Raw Mater. 2024, 12, 1–12. [Google Scholar] [CrossRef]
- Farkas, J. Physical Methods of Food Preservation. In Food Microbiology: Fundamentals and Frontiers; ASM Press: Washington, DC, USA, 2007. [Google Scholar]
- Pedreschi, F.; Mariotti-Celis, M.S. Irradiation Kills Microbes. In Genetically Modified and Irradiated Food; Elsevier: Amsterdam, The Netherlands, 2020; pp. 233–242. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef]
- Cuba, V.; Pavelková, T.; Múčka, V. Effect of Dose and Dose Rate of Gamma Radiation on Catalytic Activity of Catalase. J. Radioanal. Nucl. Chem. 2010, 286, 619–624. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Carrillo, C.; Barrio, Á.; del Mar Cavia, M.; Alonso-Torre, S. Global Antioxidant Response of Meat. J. Sci. Food Agric. 2017, 97, 2358–2365. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive Oxygen Species and Antioxidants in Postharvest Vegetables and Fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Trawczy’nska, I.; Trawczy’nska, T. New Method of Determining Kinetic Parameters for Decomposition of Hydrogen Peroxide by Catalase. Catalysts 2020, 10, 323. [Google Scholar] [CrossRef]
- Krishna, H.; Avinash, K.; Shivakumar, A.; Al-Tayar, N.G.S.; Shrestha, A.K. A Quantitative Method for the Detection and Validation of Catalase Activity at Physiological Concentration in Human Serum, Plasma and Erythrocytes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 251, 119358. [Google Scholar] [CrossRef]
- Nicholls, P.; Fita, I.; Loewen, P.C. Enzymology and Structure of Catalases. Adv. Inorg. Chem. 2000, 51, 51–106. [Google Scholar] [CrossRef]
- Hadwan, M.H. Simple Spectrophotometric Assay for Measuring Catalase Activity in Biological Tissues. BMC Biochem. 2018, 19, 7. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and Ascorbate Peroxidase—Representative H2O2-Detoxifying Heme Enzymes in Plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Vitolo, M. Decomposition of Hydrogen Peroxide by Catalase. World J. Pharm. Pharm. Sci. 2021, 10, 47–56. [Google Scholar]
- Hadwan, M.H.; Ali, S.K. New Spectrophotometric Assay for Assessments of Catalase Activity in Biological Samples. Anal. Biochem. 2018, 542, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, R.E.; Prassinos, A.J.; Osborne, J.D.; Raviv, Z.; Balish, M.F. Reduction of Hydrogen Peroxide Accumulation and Toxicity by a Catalase from Mycoplasma Iowae. PLoS ONE 2014, 9, e105188. [Google Scholar] [CrossRef] [PubMed]
- Miłek, J. Estimation of the Kinetic Parameters for H2O2 Enzymatic Decomposition and for Catalase Deactivation. Braz. J. Chem. Eng. 2018, 35, 995–1004. [Google Scholar] [CrossRef]
- Hernández, P.; López, A.; Marco, M.; Blasco, A. Influence of Muscle Type, Refrigeration Storage and Genetic Line on Antioxidant Enzyme Activity in Rabbit Meat. World Rabbit Sci. 2002, 10, 141–146. [Google Scholar]
- Kaur, G.; Sharma, S.; Das, N. Comparison of Catalase Activity in Different Organs of the Potato (Solanum tuberosum L.) Cultivars Grown under Field Condition and Purification by Three-Phase Partitioning. Acta Physiol. Plant 2020, 42, 10. [Google Scholar] [CrossRef]
- Dotsenko, O.I.; Mischenko, A.M.; Taradina, G.V. Vibration Influence on the O2-Dependent Processes Activity in Human Erythrocytes. Regul. Mech. Biosyst. 2021, 12, 452–458. [Google Scholar] [CrossRef]
- Takemoto, K.; Ozaki, A.; Yagi, M.; Ando, H. Measurement of Catalase Activity Using Catalase Inhibitors. J. Anal. Sci. Methods Instrum. 2024, 14, 39–50. [Google Scholar] [CrossRef]
- Patruno, A.; Tabrez, S.; Amerio, P.; Pesce, M.; Vianale, G.; Franceschelli, S.; Grilli, A.; Kamal, M.A.; Reale, M. Kinetic Study on the Effects of Extremely Low Frequency Electromagnetic Field on Catalase, Cytochrome P450 and Inducible Nitric Oxide Synthase in Human HaCaT and THP-1 Cell Lines. CNS Neurol. Disord. Drug Targets 2012, 10, 936–944. [Google Scholar] [CrossRef]
- Horikoshi, S.; Nakamura, K.; Kawaguchi, M.; Kondo, J.; Serpone, N. Effect of Microwave Radiation on the Activity of Catalase. Decomposition of Hydrogen Peroxide under Microwave and Conventional Heating. RSC Adv. 2016, 6, 48237–48244. [Google Scholar] [CrossRef]
- Mirmiranpour, H.; Nosrati, F.S.; Sobhani, S.O.; Takantape, S.N.; Amjadi, A. Effect of Low-Level Laser Irradiation on the Function of Glycated Catalase. J. Lasers Med. Sci. 2018, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Zigman, S.; Reddan, J.; Schultz, J.B.; McDaniel, T. Structural and Functional Changes in Catalase Induced by Near-UV Radiation. Photochem. Photobiol. 1996, 63, 818–824. [Google Scholar] [CrossRef]
- Muhlisin; Utama, D.T.; Lee, J.H.; Choi, J.H.; Lee, S.K. Antioxidant Enzyme Activity, Iron Content and Lipid Oxidation of Raw and Cooked Meat of Korean Native Chickens and Other Poultry. Asian-Australas. J. Anim. Sci. 2015, 29, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.A.; Rhee, K.S.; Hernández, P. Stability of Catalase and Its Potential Role in Lipid Oxidation in Meat. Meat Sci. 2000, 54, 385–390. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Alta’ee, A.H.; Mohammed, R.M.; Hadwan, A.M.; Al-Kawaz, H.S.; Al Talebi, Z.A. An Efficient Protocol for Quantifying Catalase Activity in Biological Samples. Bull. Natl. Res. Cent. 2024, 48, 34. [Google Scholar] [CrossRef]
- Hamza, T.A.; Hadwan, M.H. New Spectrophotometric Method for the Assessment of Catalase Enzyme Activity in Biological Tissues. Curr. Anal. Chem. 2020, 16, 1054–1062. [Google Scholar] [CrossRef]
- Switala, J.; Loewen, P.C. Diversity of Properties among Catalases. Arch. Biochem. Biophys. 2002, 401, 145–154. [Google Scholar] [CrossRef]
- Kroll, R.G.; Frears, E.R.; Bayliss, A. An Oxygen Electrode-Based Assay of Catalase Activity as a Rapid Method for Estimating the Bacterial Content of Foods. J. Appl. Bacteriol. 1989, 66, 209–217. [Google Scholar] [CrossRef]
- Iwase, T.; Tajima, A.; Sugimoto, S.; Okuda, K.I.; Hironaka, I.; Kamata, Y.; Takada, K.; Mizunoe, Y. A Simple Assay for Measuring Catalase Activity: A Visual Approach. Sci. Rep. 2013, 3, 3081. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, M.A.; Hadwan, M.H. A Precise and Simple Method for Measuring Catalase Activity in Biological Samples. Chem. Pap. 2021, 75, 1669–1678. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. Rapid Kinetic Microassay for Catalase Activity. J. Biomol. Tech. 2007, 18, 185. [Google Scholar]
- Glorieux, C.; Calderon, P.B. Catalase, a Remarkable Enzyme: Targeting the Oldest Antioxidant Enzyme to Find a New Cancer Treatment Approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef]
- Gebicka, L.; Metodieva, D.; Bachman, S. The Catalatic and Peroxidatic Activity of Irradiated Dilute Aqueous Solutions of Catalase. J. Radioanal. Nucl. Chem. Artic. 1987, 116, 77–86. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, D.; Zhang, Q.; Guo, X.; Raymond, J.L.; Roy, R.A.; Zhang, D.; Tu, J. The Influence of Droplet Concentration on Phase Change and Inertial Cavitation Thresholds Associated with Acoustic Droplet Vaporization. J. Acoust. Soc. Am. 2020, 148, EL375–EL381. [Google Scholar] [CrossRef]
- Kozlova, E.; Sherstyukova, E.; Sergunova, V.; Kozlov, A.; Gudkova, O.; Inozemtsev, V.; Chernysh, A. The Toxic Influence of Excess Free Iron on Red Blood Cells in the Biophysical Experiment: An In Vitro Study. J. Toxicol. 2022, 2022, 7113958. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.K. Enzymes: Principles and Biotechnological Applications. Essays Biochem. 2015, 59, 1. [Google Scholar] [CrossRef]
- Bisswanger, H. Enzyme Kinetics; Wiley: Hoboken, NJ, USA, 2017; ISBN 9783527342518. [Google Scholar]
- Al-Rumaih, M.M.; Al-Rumaih, M.M. Influence of Ionizing Radiation on Antioxidant Enzymes in Three Species of Trigonella. Am. J. Environ. Sci. 2008, 4, 151–156. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.; El-Beltagi, H.S.; Aly, A.A.; El-Ansary, A.E. Antioxidant Enzyme Activities and Lipid Peroxidation as Biomarker Compounds for Potato Tuber Stored by Gamma Radiation. Asian Pac. J. Trop. Biomed. 2012, 2, S1548–S1555. [Google Scholar] [CrossRef]
- Semenova, A.A.; Aslanova, M.A.; Dydykin, A.S.; Derevitskaya, O.K.; Bero, A.L.; Bagryantseva, O.V.; Nikityuk, D.B. Effect of Ionizing Radiation on Microbiological Safety and Activity of Antioxidant Enzymes in Minced Meat. Probl. Nutr. 2022, 91, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Bliznyuk, U.A.; Yu Borchegovskaya, P.; Chernyaev, A.P.; Avdukhina, V.M.; Ipatova, V.S.; Leontiev, V.A.; Studenikin, F.R. Computer Simulation to Determine Food Irradiation Dose Levels. IOP Conf. Ser. Earth Environ. Sci. 2019, 365, 012002. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ipatova, V.; Bliznyuk, U.; Borshchegovskaya, P.; Chernyaev, A.; Toropygina, M.; Kim, V.; Nikitchenko, A.; Kozlov, A.; Yurov, D.; Beklemishev, M.; et al. Assessment of Catalase Inhibition Under e-Beam Irradiation. Int. J. Mol. Sci. 2025, 26, 4358. https://doi.org/10.3390/ijms26094358
Ipatova V, Bliznyuk U, Borshchegovskaya P, Chernyaev A, Toropygina M, Kim V, Nikitchenko A, Kozlov A, Yurov D, Beklemishev M, et al. Assessment of Catalase Inhibition Under e-Beam Irradiation. International Journal of Molecular Sciences. 2025; 26(9):4358. https://doi.org/10.3390/ijms26094358
Chicago/Turabian StyleIpatova, Victoria, Ulyana Bliznyuk, Polina Borshchegovskaya, Alexander Chernyaev, Maria Toropygina, Violetta Kim, Alexander Nikitchenko, Aleksandr Kozlov, Dmitry Yurov, Mikhail Beklemishev, and et al. 2025. "Assessment of Catalase Inhibition Under e-Beam Irradiation" International Journal of Molecular Sciences 26, no. 9: 4358. https://doi.org/10.3390/ijms26094358
APA StyleIpatova, V., Bliznyuk, U., Borshchegovskaya, P., Chernyaev, A., Toropygina, M., Kim, V., Nikitchenko, A., Kozlov, A., Yurov, D., Beklemishev, M., Rodin, I., & Kozlova, E. (2025). Assessment of Catalase Inhibition Under e-Beam Irradiation. International Journal of Molecular Sciences, 26(9), 4358. https://doi.org/10.3390/ijms26094358






