Advances in Photothermal Therapy for Oral Cancer
Abstract
1. Introduction
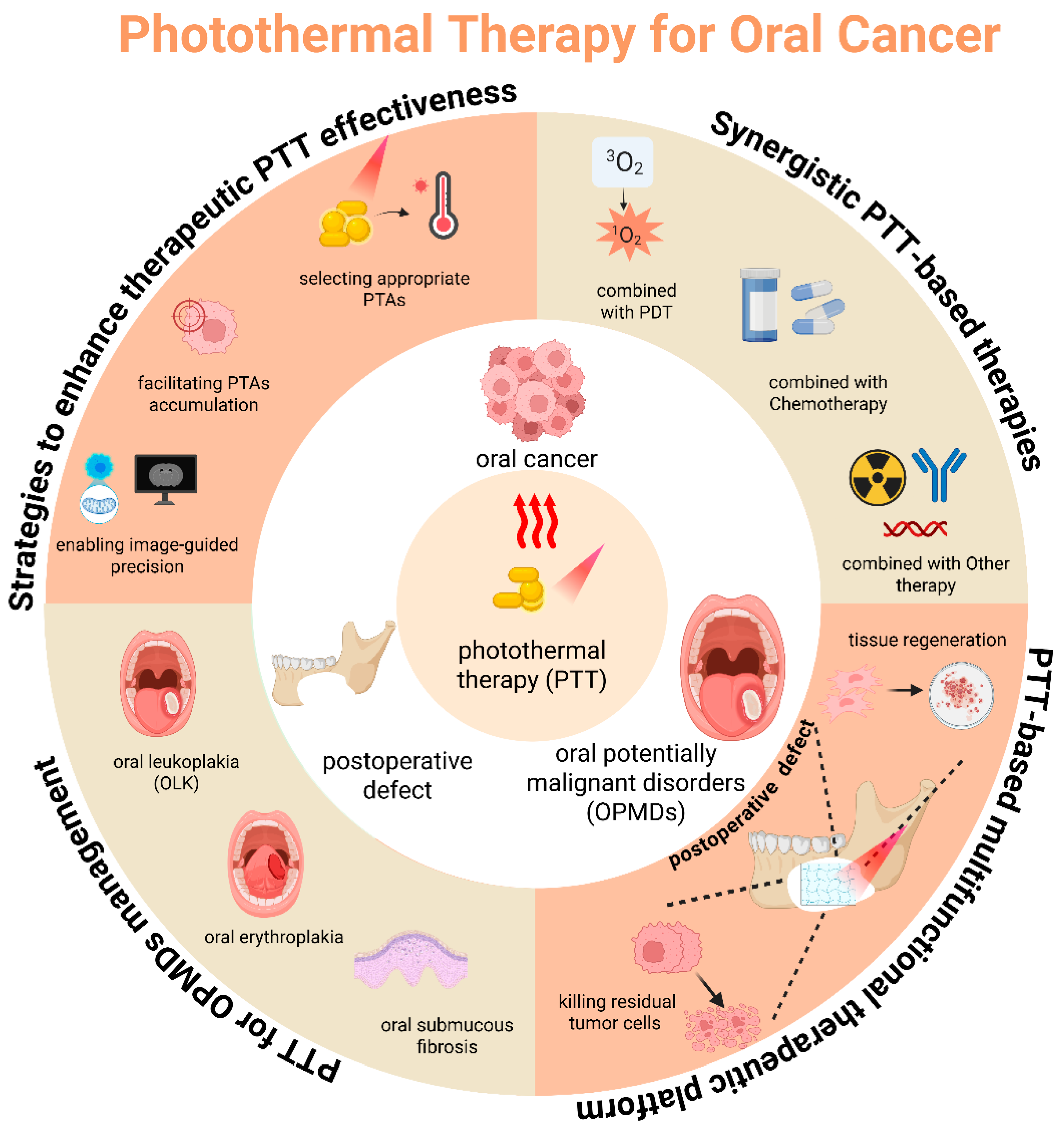
2. Strategies to Enhance PTT Therapeutic Effectiveness
2.1. Selection of Appropriate PTAs
2.2. Accumulation of PTAs in Tumor Tissue
2.3. Navigation from Imaging
3. Synergistic PTT-Based Therapies
3.1. PTT Combined with PDT
3.2. PTT Combined with Chemotherapy
3.3. PTT Combined with Radiotherapy
3.4. PTT Combined with Gene Therapy
3.5. PTT Combined with Immunotherapy
4. PTT-Based Multifunctional Therapeutic Platform
5. PTT for OPMDs Management
6. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| 4NQO | 4-nitroquinoline 1-oxide |
| APCs | Antigen-presenting cells |
| AuNPs | Au nanoparticles |
| AuNRs | Au nanorods |
| BMSCs | Bone marrow mesenchymal stem cells |
| BP | Black phosphorus |
| BSA | Bovine serum albumin |
| CDX | Cell line-derived xenograft |
| Ce6 | Chlorin e6 |
| COL | Collagen |
| CT | Computed tomography |
| CTAB | Cetyltrimethylammonium bromide |
| CTLs | Cytotoxic T lymphocytes |
| DMBA | 7,12-dimethylbenz[a]anthracene |
| DOX | Doxorubicin |
| DDS | Drug delivery systems |
| EGFR | Epithelial growth factor receptor |
| EPR | Enhanced permeability and retention |
| FAP | Fibroblast activation protein |
| FL | Fluorescence imaging |
| GNR | Gold nanorod |
| GO | Graphene oxide |
| HA | Hyaluronic acid |
| HBP | Hamster buccal pouch |
| HMPBs | Hollow meso-porous Prussian blue NPs |
| HNSCC | Head and neck squamous cell carcinoma |
| HSP | Heat shock protein |
| HSR | Heat shock response |
| ICG | Indocyanine green |
| KBV | Human oral epithelial carcinoma vincristine-resistant tumor |
| LSPR | Localized surface plasmon resonance |
| MMP | Matrix metalloproteinase |
| mPTT | Mild-temperature photothermal therapy |
| MRI | Magnetic resonance imaging |
| nHA | Nanohydroxyapatite |
| NIR | Near-infrared |
| NPs | Nanoparticles |
| NSs | Nanosheets |
| OLK | Oral leukoplakia |
| OPMDs | Oral potentially malignant disorders |
| OSCC | Oral squamous cell carcinoma |
| PA | Photoacoustic |
| PDA | Polydopamine |
| PDT | Photodynamic therapy |
| PEG | Poly(ethylene glycol) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PLTs | Platelets |
| PPTT | Plasmonic photothermal therapy |
| PTT | Photothermal therapy |
| P. gingivalis | Porphyromonas gingivalis |
| PS | Photosensitizers |
| PT | Photothermal |
| PTAs | Photothermal agents |
| PTT | Photothermal therapy |
| RB | Rose bengal |
| ROS | Reactive oxygen species |
| RT | Radiotherapy |
| SF | Silk fibroin |
| SIM | Simvastatin |
| siRNA | Small interfering RNA |
| STAT3 | Signal transducer activator of transcription 3 |
| SWCNTs | Single-walled carbon nanotubes |
| TME | Tumor microenvironment |
| VCR | Vincristine |
References
- Spanemberg, J.C.; Cardoso, J.A.; Slob, E.M.G.B.; López-López, J. Quality of life related to oral health and its impact in adults. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 234–239. [Google Scholar] [CrossRef]
- Coletta, R.D.; Yeudall, W.A.; Salo, T. Current trends on prevalence, risk factors and prevention of oral cancer. Front. Oral Health 2024, 5, 1505833. [Google Scholar] [CrossRef]
- Balaji, H.; Aithal, V.U.; Varghese, J.J.; Devaraja, K.; Kumar, A.N.N. Agreement between patient-reported and clinician-rated speech and swallowing outcomes—Understanding the trend in post-operative oral cavity cancer patients. Oral Oncol. 2024, 159, 107068. [Google Scholar] [CrossRef]
- Kijowska, J.; Grzegorczyk, J.; Gliwa, K.; Jędras, A.; Sitarz, M. Epidemiology, Diagnostics, and Therapy of Oral Cancer—Update Review. Cancers 2024, 16, 3156. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA-Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tota, J.E.; Engels, E.A.; Lingen, M.W.; Agrawal, N.; Kerr, A.R.; Zumsteg, Z.S.; Cheung, L.C.; Katki, H.A.; Abnet, C.C.; Chaturvedi, A.K. Inflammatory Tongue Conditions and Risk of Oral Tongue Cancer Among the US Elderly Individuals. J. Clin. Oncol. 2024, 42, 1745–1753. [Google Scholar] [CrossRef]
- Rumgay, H.; Nethan, S.T.; Shah, R.; Vignat, J.; Ayo-Yusuf, O.; Chaturvedi, P.; Guerra, E.N.S.; Gupta, P.C.; Gupta, R.; Liu, S.; et al. Global burden of oral cancer in 2022 attributable to smokeless tobacco and areca nut consumption: A population attributable fraction analysis. Lancet Oncol. 2024, 25, 1413–1423. [Google Scholar] [CrossRef]
- Zahiruddin, Q.S.; Jena, D.; Ballal, S.; Kumar, S.; Bhat, M.; Sharma, S.; Kumar, M.R.; Rustagi, S.; Gaidhane, A.M.; Jain, L.; et al. Burden of oral cancer and associated risk factors at national and state levels: A systematic analysis from the global burden of disease in India, 1990–2021. Oral Oncol. 2024, 159, 107063. [Google Scholar]
- Piemonte, E.D.; Lazos, J.P.; Gilligan, G.M.; Panico, R.L.; Werner, L.C.; Yang, Y.-H.; Warnakulasuriya, S. Chronic mechanical irritation enhances the effect of tobacco and alcohol on the risk of oral squamous cell carcinoma: A case-control study in Argentina. Clin. Oral Investig. 2022, 26, 6317–6326. [Google Scholar] [CrossRef]
- Nokovitch, L.; Maquet, C.; Crampon, F.; Taihi, I.; Roussel, L.-M.; Obongo, R.; Virard, F.; Fervers, B.; Deneuve, S. Oral Cavity Squamous Cell Carcinoma Risk Factors: State of the Art. J. Clin. Med. 2023, 12, 3264. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Oral potentially malignant disorders: A comprehensive review on clinical aspects and management. Oral Oncol. 2020, 102, 104550. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Villa, A.; Lodolo, M.; Ha, P. Oncological Outcomes of Patients With Oral Potentially Malignant Disorders. JAMA Otolaryngol.–Head Neck Surg. 2025, 151, 65–71. [Google Scholar] [CrossRef]
- Kerr, A.R.; Lodi, G. Management of oral potentially malignant disorders. Oral Dis. 2021, 27, 2008–2025. [Google Scholar] [CrossRef]
- Neville, B.W.; Day, T.A. Oral cancer and precancerous lesions. CA-Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral squamous cell carcinomas: State of the field and emerging directions. Int. J. Oral Sci. 2023, 15, 44. [Google Scholar] [CrossRef]
- Matos, L.L.; Guimarães, Y.L.M.; Leite, A.K.; Cernea, C.R. Management of Stage III Oral Cavity Squamous Cell Carcinoma in Light of the New Staging System: A Critical Review. Curr. Oncol. Rep. 2023, 25, 107–113. [Google Scholar] [CrossRef]
- Fan, H.-Y.; Zhu, Z.-L.; Zhang, W.-L.; Yin, Y.-J.; Tang, Y.-L.; Liang, X.-H.; Zhang, L. Light stimulus responsive nanomedicine in the treatment of oral squamous cell carcinoma. Eur. J. Med. Chem. 2020, 199, 112394. [Google Scholar] [CrossRef]
- Pan, Q.; Tang, H.; Xie, L.; Zhu, H.; Wu, D.; Liu, R.; He, B.; Pu, Y. Recent advances in phototherapeutic nanosystems for oral cancer. J. Mater. Chem. B 2024, 12, 11560–11572. [Google Scholar] [CrossRef]
- Shamsi, U.; Khan, M.A.A.; Qadir, M.S.; Rehman, S.S.U.; Azam, I.; Idress, R. Factors associated with the survival of oral cavity cancer patients: A single institution experience from Karachi, Pakistan. BMC Oral Health 2024, 24, 1427. [Google Scholar] [CrossRef]
- Wei, L.-Y.; Li, Z.-Z.; Xu, Z.-Y.; Wang, G.-R.; Xiao, Y.; Liu, B.; Bu, L.-L. The ending is not the end: Lymph node metastasis in oral squamous cell carcinoma. Int. Immunopharmacol. 2025, 146, 113917. [Google Scholar] [CrossRef]
- Alfertshofer, M.; Knoedler, L.; Mrosk, F.; Schmitz, A.; Richter, M.; Rendenbach, C.; Doll, C.; Heiland, M.; Koerdt, S. Histopathological invasion patterns and prognosis in Oral Squamous Cell Carcinoma: A retrospective analysis of 560 cases. Oral Oncol. 2025, 163, 107247. [Google Scholar] [CrossRef]
- Li, S.S.; Wu, C.Z.; Li, L.J. Progress on photodynamic therapy in oral diseases. West China J. Stomatol. 2021, 39, 215–220. [Google Scholar]
- Wu, Y.; Li, X.; Liu, H.; Yang, X.; Li, R.; Zhao, H.; Shang, Z. Organoids in the oral and maxillofacial region: Present and future. Int. J. Oral Sci. 2024, 16, 61. [Google Scholar] [CrossRef]
- Cheng, Q.-S.; Xu, P.-Y.; Luo, S.-C.; Chen, A.-Z. Advances in Adhesive Materials for Oral and Maxillofacial Soft Tissue Diseases. Macromol. Biosci. 2025, 25, 2400494. [Google Scholar] [CrossRef]
- Chamoli, A.; Gosavi, A.S.; Shirwadkar, U.P.; Wangdale, K.V.; Behera, S.K.; Kurrey, N.K.; Kalia, K.; Mandoli, A. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral Oncol. 2021, 121, 105451. [Google Scholar] [CrossRef]
- Gates, J.C.; Abouyared, M.; Shnayder, Y.; Farwell, D.G.; Day, A.; Alawi, F.; Moore, M.; Holcomb, A.J.; Birkeland, A.; Epstein, J. Clinical Management Update of Oral Leukoplakia: A Review From the American Head and Neck Society Cancer Prevention Service. Head Neck-J. Sci. Spec. Head Neck 2025, 47, 733–741. [Google Scholar] [CrossRef]
- Niu, Q.; Sun, Q.; Bai, R.; Zhang, Y.; Zhuang, Z.; Zhang, X.; Xin, T.; Chen, S.; Han, B. Progress of Nanomaterials-Based Photothermal Therapy for Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 10428. [Google Scholar] [CrossRef]
- Zhuoping, Z.; Pengfei, X.; Yang, G.; Caifeng, Z.; Kuanshou, Z.; Qingmei, L. Research progress on the use of photothermal therapy to treat oral squamous cell carcinoma. Int. J. Stomatol. 2022, 49, 462–470. [Google Scholar]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef]
- Cao, L.; Wu, Y.; Shan, Y.; Tan, B.; Liao, J. A review: Potential application and outlook of photothermal therapy in oral cancer treatment. Biomed. Mater. 2022, 17, 022008. [Google Scholar] [CrossRef]
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, J.; Zhou, Y.; Zhang, Z.; Guo, L.; Bi, X. Prediction of postoperative dysphagia in patients with oral cancer: A prospective cohort study. J. Stomatol. Oral Maxillofac. Surg. 2024, 125 (Suppl. S2), 101957. [Google Scholar] [CrossRef]
- Shirakawa, J.; Kaneuji, T.; Matsuno, D.; Nagata, J.; Hirayama, B.; Tanaka, F.; Nakamura, Y.; Yamashita, Y. Correlation during the extent of surgical resection, oral function and quality of life after tongue cancer surgery: Single-institution study. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101907. [Google Scholar] [CrossRef]
- Goetz, J.W.; Rabinowits, G.; Kalman, N.; Villa, A. A Review of Immunotherapy for Head and Neck Cancer. J. Dent. Res. 2024, 103, 1185–1196. [Google Scholar] [CrossRef]
- Mitea, G.; Schröder, V.; Iancu, I.M.; Mireșan, H.; Iancu, V.; Bucur, L.A.; Badea, F.C. Molecular Targets of Plant-Derived Bioactive Compounds in Oral Squamous Cell Carcinoma. Cancers 2024, 16, 3612. [Google Scholar] [CrossRef]
- Chen, J.; Xu, X.; Wang, K.; Xue, M.; Wang, Q.; Zhong, W.; Wan, Y.; Liu, X.; Zheng, J.; Gao, G.; et al. Hypoxia-Activated Liposomes Enable Synergistic Photodynamic Therapy for Oral Cancer. Adv. Healthc. Mater. 2025, 14, e2404395. [Google Scholar] [CrossRef]
- Nandini, D.B.; Rao, R.S.; Hosmani, J.; Khan, S.; Patil, S.; Awan, K.H. Novel therapies in the management of oral cancer: An update. Dis. Mon. 2020, 66, 101036. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Z.; Yang, J.; Xu, R.; Deng, H.; Ma, S.; Fang, T.; Zhang, J.; Shen, Q. Reactive oxygen species/photothermal therapy dual-triggered biomimetic gold nanocages nanoplatform for combination cancer therapy via ferroptosis and tumor-associated macrophage repolarization mechanism. J. Colloid Interface Sci. 2022, 606, 1950–1965. [Google Scholar] [CrossRef]
- Yu, S.; Xia, G.; Yang, N.; Yuan, L.; Li, J.; Wang, Q.; Li, D.; Ding, L.; Fan, Z.; Li, J. Noble Metal Nanoparticle-Based Photothermal Therapy: Development and Application in Effective Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 5632. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.; Yang, G.; Yang, P.; He, F.; Lin, J.; Jin, D.; Xing, B. Recent advances in functional nanomaterials for light–triggered cancer therapy. Nano Today 2018, 19, 146–187. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Ren, S.; Cheng, X.; Chen, M.; Liu, C.; Zhao, P.; Huang, W.; He, J.; Zhou, Z.; Miao, L. Hypotoxic and Rapidly Metabolic PEG-PCL-C3-ICG Nanoparticles for Fluorescence-Guided Photothermal/Photodynamic Therapy against OSCC. ACS Appl. Mater. Interfaces 2017, 9, 31509–31518. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Feng, X.; Liang, H.; Wang, K.; Song, Y.; Tan, L.; Wang, B.; Luo, R.; Liao, Z.; Li, G.; et al. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mater. Today 2020, 36, 48–62. [Google Scholar] [CrossRef]
- Wu, M.; Liu, H.; Li, D.; Zhu, Y.; Wu, P.; Chen, Z.; Chen, F.; Chen, Y.; Deng, Z.; Cai, L. Smart-Responsive Multifunctional Therapeutic System for Improved Regenerative Microenvironment and Accelerated Bone Regeneration via Mild Photothermal Therapy. Adv. Sci. 2024, 11, 2304641. [Google Scholar] [CrossRef]
- Lan, J.; Zeng, R.; Li, Z.; Yang, X.; Liu, L.; Chen, L.; Sun, L.; Shen, Y.; Zhang, T.; Ding, Y. Biomimetic Nanomodulators With Synergism of Photothermal Therapy and Vessel Normalization for Boosting Potent Anticancer Immunity. Adv. Mater. 2024, 36, 2408511. [Google Scholar] [CrossRef]
- Gao, J.; Qin, H.; Wang, F.; Liu, L.; Tian, H.; Wang, H.; Wang, S.; Ou, J.; Ye, Y.; Peng, F.; et al. Hyperthermia-triggered biomimetic bubble nanomachines. Nat. Commun. 2023, 14, 4867. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Z.; Yu, X.; Yu, C.; Sui, S.; Zhang, C.; Bao, C.; Zeng, X.; Chen, Q.; Peng, Q. Intratumor delivery of amino-modified graphene oxide as a multifunctional photothermal agent for efficient antitumor phototherapy. J. Colloid Interface Sci. 2023, 652, 1108–1116. [Google Scholar] [CrossRef]
- Jiang, Y.; Tan, Z.; Zhao, T.; Wu, J.; Li, Y.; Jia, Y.; Peng, Z. Indocyanine green derived carbon dots with significantly enhanced properties for efficient photothermal therapy. Nanoscale 2023, 15, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, P.; Zhang, J.; Lin, Z.; Bai, L.; Shen, H. Applications of nanotheranostics in the second near-infrared window in bioimaging and cancer treatment. Nanoscale 2024, 16, 21697–21730. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Hao, M.; Li, X.; Zhang, X.; Tao, B.; Shi, H.; Wu, J.; Li, Y.; Li, X.; Li, S.; Wu, H.; et al. Tongue squamous cell carcinoma-targeting Au-HN-1 nanosystem for CT imaging and photothermal therapy. Int. J. Oral Sci. 2025, 17, 9. [Google Scholar] [CrossRef]
- Li, H.; Zhu, L.; Zhang, Y.; Yang, L.; Wu, W.; Yang, D. Biomimetic nanotherapeutics for homotypic-targeting photothermal/chemotherapy of oral cancer. J. Control. Release 2024, 366, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yan, Y.; Wang, Y.; Fan, Y.; Zou, H.; Liu, H.; Luo, R.; Li, R.; Liu, H. A bifunctional MXene-modified scaffold for photothermal therapy and maxillofacial tissue regeneration. Regen. Biomater. 2021, 8, rbab057. [Google Scholar] [CrossRef]
- Luo, R.; Li, F.; Wang, Y.; Zou, H.; Shang, J.; Fan, Y.; Liu, H.; Xu, Z.; Li, R.; Liu, H. MXene-modified 3D printed scaffold for photothermal therapy and facilitation of oral mucosal wound reconstruction. Mater. Des. 2023, 227, 111731. [Google Scholar] [CrossRef]
- Fan, Y.; Li, F.; Zou, H.; Xu, Z.; Liu, H.; Luo, R.; Zhang, G.; Li, R.; Yan, Y.; Liu, H. Photothermal effect of indocyanine green modified scaffold inhibits oral squamous cell carcinoma and promotes wound healing. Biomater. Adv. 2022, 137, 212811. [Google Scholar] [CrossRef] [PubMed]
- Mackey, M.A.; Ali, M.R.K.; Austin, L.A.; Near, R.D.; El-Sayed, M.A. The Most Effective Gold Nanorod Size for Plasmonic Photothermal Therapy: Theory and In Vitro Experiments. J. Phys. Chem. B 2014, 118, 1319–1326. [Google Scholar] [CrossRef]
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.; El-Sayed, I.H.; Chu, H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008, 269, 57–66. [Google Scholar] [CrossRef]
- Mehdizadeh, A.; Pandesh, S.; Shakeri-Zadeh, A.; Kamrava, S.K.; Habib-Agahi, M.; Farhadi, M.; Pishghadam, M.; Ahmadi, A.; Arami, S.; Fedutik, Y. The effects of folate-conjugated gold nanorods in combination with plasmonic photothermal therapy on mouth epidermal carcinoma cells. Lasers Med. Sci. 2013, 29, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-T.; Liu, C.-H.; Chin, Y.; Chen, S.-Y.; Liu, S.H.; Hsu, Y.-C.; Wu, K.C.W. Biocompatible and multifunctional gold nanorods for effective photothermal therapy of oral squamous cell carcinoma. J. Mater. Chem. B 2019, 7, 4451–4460. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.-L.; Ma, L.; Wang, W.; Liu, H.; Wan, D.; Liu, J.-F.; Li, A.; Guo, S.-S.; Zhang, L.; et al. Platelet-Facilitated Photothermal Therapy of Head and Neck Squamous Cell Carcinoma. Angew. Chem. Int. Ed. 2018, 57, 986–991. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Afifi, M.M.; El Sheikh, S.M.; Abdelsalam, M.M.; Ramadan, H.; Omar, T.A.; El Tantawi, M.; Abdel-Razek, K.M.; Mohamed, M. Therapeutic efficacy of plasmonic photothermal nanoparticles in hamster buccal pouch carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 743–751. [Google Scholar] [CrossRef]
- Elsayed, I.; Huang, X.; Elsayed, M. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef]
- Chen, D.; Chen, Z.; Wang, Z.; Yang, Y.; Jiang, Y.; Hu, C. Photothermal effect of nano-copper sulfide against tongue squamous cell carcinoma. J. South. Med. Univ. 2021, 41, 1843–1849. [Google Scholar]
- Su, J.; Lu, S.; Jiang, S.; Li, B.; Liu, B.; Sun, Q.; Li, J.; Wang, F.; Wei, Y. Engineered Protein Photo-Thermal Hydrogels for Outstanding In Situ Tongue Cancer Therapy. Adv. Mater. 2021, 33, 2100619. [Google Scholar] [CrossRef]
- Fekrazad, R.; Hakimiha, N.; Farokhi, E.; Rasaee, M.J.; Ardestani, M.S.; Kalhori, K.A.; Sheikholeslami, F. Treatment of oral squamous cell carcinoma using anti-HER2 immunonanoshells. Int. J. Nanomed. 2011, 6, 2749–2755. [Google Scholar] [CrossRef][Green Version]
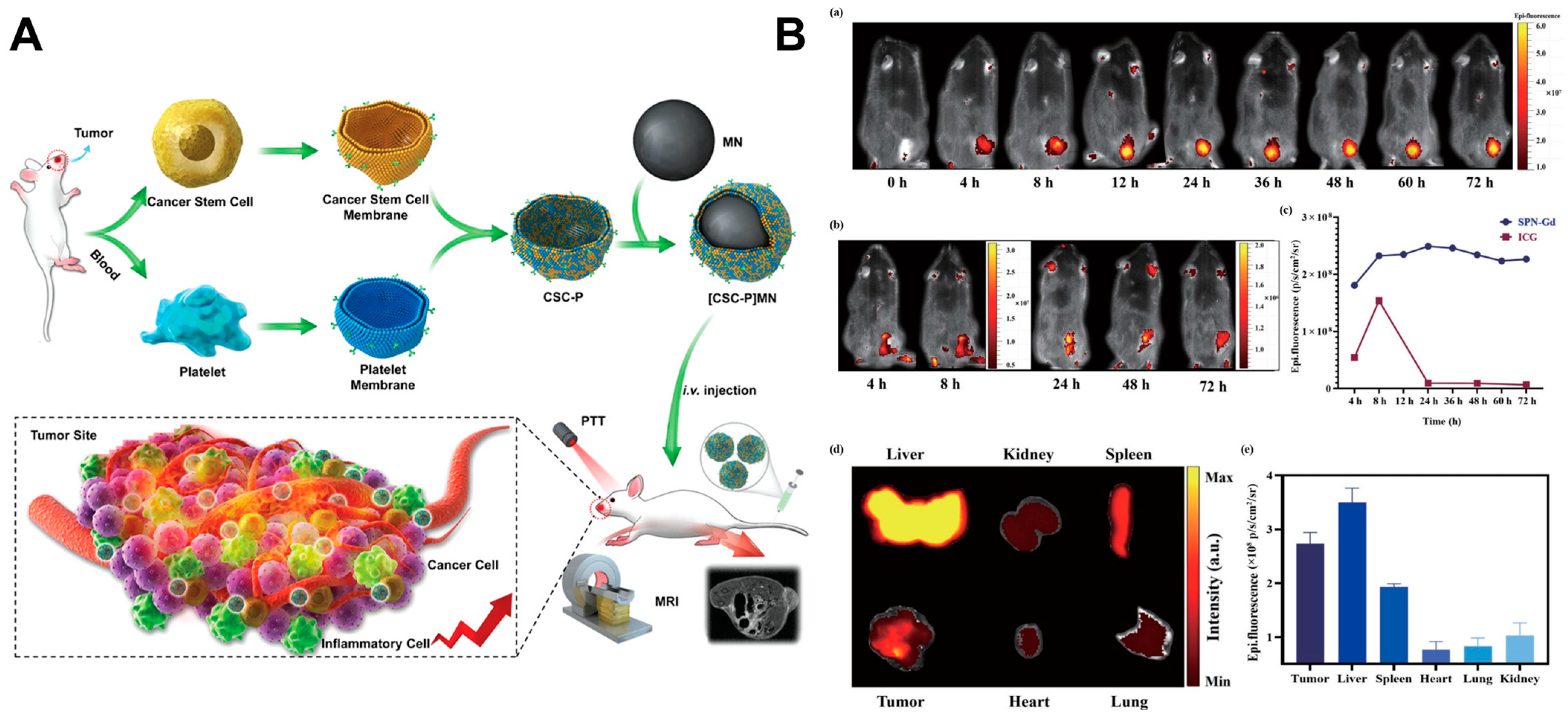
- Bu, L.-L.; Rao, L.; Yu, G.-T.; Chen, L.; Deng, W.-W.; Liu, J.-F.; Wu, H.; Meng, Q.-F.; Guo, S.-S.; Zhao, X.-Z.; et al. Cancer Stem Cell-Platelet Hybrid Membrane-Coated Magnetic Nanoparticles for Enhanced Photothermal Therapy of Head and Neck Squamous Cell Carcinoma. Adv. Funct. Mater. 2019, 29, 1807733. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, L.; Huang, X.; Lin, J.; Gao, J.; Yang, G.; Wu, Y.; Wang, C.; Kang, X.; Yao, Y.; et al. A biomimetic nanoplatform for customized photothermal therapy of HNSCC evaluated on patient-derived xenograft models. Int. J. Oral Sci. 2023, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Q.; Zhang, Y.; Yang, Y.; Zhou, X.; Peng, W.; Liang, Z.; Zeng, X.; Wang, Q.; Gao, N. Charge-reversal nanomedicine based on black phosphorus for the development of A Novel photothermal therapy of oral cancer. Drug Deliv. 2021, 28, 700–708. [Google Scholar] [CrossRef]
- Das, R.K.; Panda, S.; Bhol, C.S.; Bhutia, S.K.; Mohapatra, S. N-Doped Carbon Quantum Dot (NCQD)-Deposited Carbon Capsules for Synergistic Fluorescence Imaging and Photothermal Therapy of Oral Cancer. Langmuir 2019, 35, 15320–15329. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Gao, A.; Hu, Y.; Hu, Z.; Xie, C.; Lin, Z. Gadolinium-containing semiconducting polymer nanoparticles for magnetic resonance/fluorescence dual-modal imaging and photothermal therapy of oral squamous cell carcinoma. Nano Res. 2023, 16, 2808–2820. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, Y.; Li, Y.; Song, L.; Lin, Y.; Liu, K.; Zhang, Y.; Zvyagin, A.V.; Fang, L.; Sun, Y.; et al. Au/Mn nanodot platform for in vivo CT/MRI/FI multimodal bioimaging and photothermal therapy against tongue cancer. J. Mater. Chem. B 2023, 11, 4752–4762. [Google Scholar] [CrossRef] [PubMed]
- Men, C.; Zhang, Y.; Shi, P.; Tang, Z.; Cheng, X. alphanubeta3 integrin-targeted ICG-derived probes for imaging-guided surgery and photothermal therapy of oral cancer. Analyst 2023, 148, 6334–6340. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, C.; Lin, X.; Sun, R.; Li, Z.; Chen, S.; Liu, Y.; Wu, J.; Yu, Z.; Liu, X. Tunable Nanoparticles with Aggregation-Induced Emission Heater for Precise Synergistic Photothermal and Thermodynamic Oral Cancer Therapy of Patient-Derived Tumor Xenograft. Adv. Sci. 2023, 10, 2205780. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.-H.; Liu, Q.; Huang, H.; Chen, M.; Li, K.; Li, C.; Yu, X.-F.; Chu, P.K. Rose-bengal-conjugated gold nanorods for in vivo photodynamic and photothermal oral cancer therapies. Biomaterials 2014, 35, 1954–1966. [Google Scholar] [CrossRef]
- Song, W.; Li, Y.; Wang, Y.; Wang, D.; He, D.; Chen, W.; Yin, W.; Yang, W. Indocyanine Green-Loaded Gold Nanoflowers@Two Layers of Silica Nanocomposites for Photothermal and Photodynamic Therapy of Oral Carcinoma. J. Biomed. Nanotechnol. 2017, 13, 1115–1123. [Google Scholar] [CrossRef]
- Chu, C.-K.; Tu, Y.-C.; Hsiao, J.-H.; Yu, J.-H.; Yu, C.-K.; Chen, S.-Y.; Tseng, P.-H.; Chen, S.; Kiang, Y.-W.; Yang, C.C. Combination of photothermal and photodynamic inactivation of cancer cells through surface plasmon resonance of a gold nanoring. Nanotechnology 2016, 27, 115102. [Google Scholar] [CrossRef]
- Bhana, S.; Lin, G.; Wang, L.; Starring, H.; Mishra, S.R.; Liu, G.; Huang, X. Near-Infrared-Absorbing Gold Nanopopcorns with Iron Oxide Cluster Core for Magnetically Amplified Photothermal and Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2015, 7, 11637–11647. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Deng, G.; Han, Y.; Yang, G.; Zou, R.; Zhang, Z.; Sun, S.; Hu, J. Right Cu2−xS@MnS Core–Shell Nanoparticles as a Photo/H2O2-Responsive Platform for Effective Cancer Theranostics. Adv. Sci. 2019, 6, 1901461. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, F.; Tian, R.; Zhang, L.; Fu, G.; Yang, L.; Zhu, L. Nanotubes-Embedded Indocyanine Green–Hyaluronic Acid Nanoparticles for Photoacoustic-Imaging-Guided Phototherapy. ACS Appl. Mater. Interfaces 2016, 8, 5608–5617. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, X.; Wang, L.; Zhou, X.; Yang, X.; Zhang, Z.; Huang, X. Versatile cobalt-glycerate nanoplatform for MR-guided neoadjuvant photo-therapy of oral squamous cell carcinoma. Chem. Eng. J. 2022, 437, 135476. [Google Scholar] [CrossRef]
- Li, X.; Hao, M.; Liu, A.; Li, L.; Nešić, M.D.; Yang, B.; Liu, W.; Lin, Q. Dual-activity nanozyme as an oxygen pump to alleviate tumor hypoxia and enhance photodynamic/NIR-II photothermal therapy for sniping oral squamous cell carcinoma. Acta Biomater. 2024, 190, 476–487. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, D.; Wang, L.; Sun, Y.; Li, J.J.; Hill, B.; Yang, F.; Li, Y.; Lam, K.S. Unique Photochemo-Immuno-Nanoplatform against Orthotopic Xenograft Oral Cancer and Metastatic Syngeneic Breast Cancer. Nano Lett. 2018, 18, 7092–7103. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, F.; Huang, N.; Li, J.; Wu, C.; Tan, B.; Liu, Y.; Li, L.; Yang, C.; Shao, D.; et al. Near-infrared light-responsive hybrid hydrogels for the synergistic chemo-photothermal therapy of oral cancer. Nanoscale 2021, 13, 17168–17182. [Google Scholar] [CrossRef]
- XIE, X.; SHAN, Y.; ZHANG, X.; WU, Y.; LIAO, J. Hyaluronic acid microneedles loaded with curcumin nanodrugs and new indocyanine green inhibits human tongue squamous carcinoma cells in vitro. J. Zhejiang Univ. Med. Sci. 2022, 51, 585–593. [Google Scholar] [CrossRef]
- Darwish, W.M.; Abdoon, A.S.; Shata, M.S.; Elmansy, M. Vincristine-loaded polymeric corona around gold nanorods for combination (chemo-photothermal) therapy of oral squamous carcinoma. React. Funct. Polym. 2020, 151, 104575. [Google Scholar] [CrossRef]
- El-Sherbiny, R.H.; Hassan, M.M.; El-Hossary, W.H.; Shata, M.S.; Darwish, W.M. Folate-targeted polymeric nanoparticles for efficient dual (chemo-photothermal) therapy of oral squamous carcinoma. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 414–424. [Google Scholar] [CrossRef]
- Mapanao, A.K.; Santi, M.; Voliani, V. Combined chemo-photothermal treatment of three-dimensional head and neck squamous cell carcinomas by gold nano-architectures. J. Colloid Interface Sci. 2021, 582, 1003–1011. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, J.; Zhu, B.; Xu, Q. Development of a multifunctional gold nanoplatform for combined chemo-photothermal therapy against oral cancer. Nanomedicine 2020, 15, 661–676. [Google Scholar] [CrossRef]
- Li, R.; Liu, C.; Wan, C.; Liu, T.; Zhang, R.; Du, J.; Wang, X.; Jiao, X.; Gao, R.; Li, B. A Targeted and pH-Responsive Nano-Graphene Oxide Nanoparticle Loaded with Doxorubicin for Synergetic Chemo-Photothermal Therapy of Oral Squamous Cell Carcinoma. Int. J. Nanomed. 2023, 18, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wan, C.; Li, Y.; Jiao, X.; Liu, T.; Gu, Y.; Gao, R.; Liu, J.; Li, B. Nanocarrier-based drug delivery system with dual targeting and NIR/pH response for synergistic treatment of oral squamous cell carcinoma. Colloids Surf. B-Biointerfaces 2024, 244, 114179. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Song, Z.; Gu, Y.; Jiao, X.; Wan, C.; Liu, T.; Zhang, R.; Gao, R.; Wang, X. A Graphene-Based Lipid Modulation Nanoplatform for Synergetic Lipid Starvation/Chemo/Photothermal Therapy of Oral Squamous Cell Carcinoma. Int. J. Nanomed. 2024, 19, 11235–11255. [Google Scholar] [CrossRef] [PubMed]
- Anup, N.; Gadeval, A.; Mule, S.R.; Gupta, T.; Tekade, R.K. Plasmonic laser-responsive BioDissolve 3D-printed graphene@cisplatin-implant for prevention of post-surgical relapse of oral cancer. Int. J. Pharm. 2024, 657, 124123. [Google Scholar] [CrossRef]
- Lin, M.; Wang, D.; Liu, S.; Huang, T.; Sun, B.; Cui, Y.; Zhang, D.; Sun, H.; Zhang, H.; Sun, H.; et al. Cupreous Complex-Loaded Chitosan Nanoparticles for Photothermal Therapy and Chemotherapy of Oral Epithelial Carcinoma. ACS Appl. Mater. Interfaces 2015, 7, 20801–20812. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Li, S.; Meng, X.; Sun, B.; Zhang, X.; Zhang, L.; Liu, Y.; Lin, M.; Zhang, H.; et al. Multidrug resistant tumors-aimed theranostics on the basis of strong electrostatic attraction between resistant cells and nanomaterials. Biomater. Sci. 2019, 7, 4990–5001. [Google Scholar] [CrossRef]
- Song, W.; Gong, J.; Wang, Y.; Zhang, Y.; Zhang, H.; Zhang, W.; Zhang, H.; Liu, X.; Zhang, T.; Yin, W.; et al. Gold nanoflowers with mesoporous silica as “nanocarriers” for drug release and photothermal therapy in the treatment of oral cancer using near-infrared (NIR) laser light. J. Nanopart. Res. 2016, 18, 101. [Google Scholar] [CrossRef]
- Xu, Y.; Hao, Y.; Li, W.; Xiao, Y.; Zhou, T.; Hu, D.; Liu, Q.; Zhou, X.; Qian, Z. Near-Infrared Responsive Doxorubicin Loaded Hollow Mesoporous Prussian Blue Nanoparticles Combined with Dissolvable Hyaluronic Acid Microneedle System for Human Oral Squamous Cell Carcinoma Therapy. J. Biomed. Nanotechnol. 2020, 16, 721–738. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Wang, F.; Yang, M.; Xie, L.; Zeng, X. Biosafety, Nontoxic Nanoparticles for VL–NIR Photothermal Therapy Against Oral Squamous Cell Carcinoma. ACS Omega 2021, 6, 11240–11247. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Xie, J.; Yang, X.; Tian, Y.; Yuan, P.; Bai, Y.; Liu, S.; Cai, B.; Chen, X. A tumor-targeted nanoplatform with stimuli-responsive cascaded activities for multiple model tumor therapy. Biomater. Sci. 2020, 8, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Neshastehriz, A.; Tabei, M.; Maleki, S.; Eynali, S.; Shakeri-Zadeh, A. Photothermal therapy using folate conjugated gold nanoparticles enhances the effects of 6 MV X-ray on mouth epidermal carcinoma cells. J. Photochem. Photobiol. B Biol. 2017, 172, 52–60. [Google Scholar] [CrossRef]
- Wang, B.-K.; Yu, X.-F.; Wang, J.-H.; Li, Z.-B.; Li, P.-H.; Wang, H.; Song, L.; Chu, P.K.; Li, C. Gold-nanorods-siRNA nanoplex for improved photothermal therapy by gene silencing. Biomaterials 2016, 78, 27–39. [Google Scholar] [CrossRef]
- Bu, L.-L.; Wang, H.-Q.; Pan, Y.; Chen, L.; Wu, H.; Wu, X.; Zhao, C.; Rao, L.; Liu, B.; Sun, Z.-J. Gelatinase-sensitive nanoparticles loaded with photosensitizer and STAT3 inhibitor for cancer photothermal therapy and immunotherapy. J. Nanobiotechnol. 2021, 19, 379. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Liu, T.; Song, C.; Wei, Z.; Tang, C.; Cao, Z.; Zou, H.; Zhang, X.; Cai, Y.; Han, W. Rhythm Mild-Temperature Photothermal Therapy Enhancing Immunogenic Cell Death Response in Oral Squamous Cell Carcinoma. Adv. Healthc. Mater. 2022, 12, 2202360. [Google Scholar] [CrossRef]
- Qian, M.; Cheng, Z.; Luo, G.; Galluzzi, M.; Shen, Y.; Li, Z.; Yang, H.; Yu, X.F. Molybdenum Diphosphide Nanorods with Laser-Potentiated Peroxidase Catalytic/Mild-Photothermal Therapy of Oral Cancer. Adv. Sci. 2021, 9, 2101527. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Guo, Z.; Hu, Z.; Yin, Y.; Duan, S.; Jia, W.; Lu, W.; Hu, J. Fe3O4 Nanoparticles That Modulate the Polarisation of Tumor-Associated Macrophages Synergize with Photothermal Therapy and Immunotherapy (PD-1/PD-L1 Inhibitors) to Enhance Anti-Tumor Therapy. Int. J. Nanomed. 2024, 19, 7185–7200. [Google Scholar] [CrossRef]
- Bai, L.; Yang, M.; Wu, J.; You, R.; Chen, Q.; Cheng, Y.; Qian, Z.; Yang, X.; Wang, Y.; Liu, Y. An injectable adhesive hydrogel for photothermal ablation and antitumor immune activation against bacteria-associated oral squamous cell carcinoma. Acta Biomater. 2024, 186, 229–245. [Google Scholar] [CrossRef]
- Zeng, J.-j.; Tang, Z.-g.; Zou, J.; Yu, J.-g. Black phosphorous nanosheets–gold nanoparticles–cisplatin for photothermal/photodynamic treatment of oral squamous cell carcinoma. Trans. Nonferrous Met. Soc. China 2021, 31, 2812–2822. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, D.; Pan, J.; Xia, C.; Fan, L.; Pu, Y.; Zhang, Q.; Ni, Y.H.; Wang, J.; Hu, Q. A near infrared light-triggered human serum albumin drug delivery system with coordination bonding of indocyanine green and cisplatin for targeting photochemistry therapy against oral squamous cell cancer. Biomater. Sci. 2019, 7, 5270–5282. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, H.; Zou, H.; Song, C.; Zhao, S.; Cao, Z.; Zhang, X.; Zhang, G.; Cai, Y.; Han, W. A novel second near-infrared theranostic agent: A win–win strategy of tracing and blocking tumor-associated vessels for oral squamous cell carcinoma. Mater. Today Nano 2022, 17, 100172. [Google Scholar] [CrossRef]
- Xue, X.; Huang, Y.; Bo, R.; Jia, B.; Wu, H.; Yuan, Y.; Wang, Z.; Ma, Z.; Jing, D.; Xu, X.; et al. Trojan Horse nanotheranostics with dual transformability and multifunctionality for highly effective cancer treatment. Nat. Commun. 2018, 9, 3653. [Google Scholar] [CrossRef]
- Shi, S.; Wang, Y.; Wang, B.; Chen, Q.; Wan, G.; Yang, X.; Zhang, J.; Zhang, L.; Li, C.; Wang, Y. Homologous-targeting biomimetic nanoparticles for photothermal therapy and Nrf2-siRNA amplified photodynamic therapy against oral tongue squamous cell carcinoma. Chem. Eng. J. 2020, 388, 124268. [Google Scholar] [CrossRef]
- Song, C.; Zhang, X.; Cao, Z.; Wei, Z.; Zhou, M.; Wang, Y.; Han, S.; Cai, Y.; Han, W. Regulating tumor cholesterol microenvironment to enhance photoimmunotherapy in oral squamous cell carcinoma. Chem. Eng. J. 2023, 462, 142160. [Google Scholar] [CrossRef]
- Chen, Q.; Shan, T.; Liang, Y.; Xu, Y.; Shi, E.; Wang, Y.; Li, C.; Wang, Y.; Cao, M. A biomimetic phototherapeutic nanoagent based on bacterial double-layered membrane vesicles for comprehensive treatment of oral squamous cell carcinoma. J. Mater. Chem. B 2023, 11, 11265–11279. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yin, Q.; Zhang, H.; Zhang, J.; Yang, G.; Weng, L.; Liu, T.; Xu, C.; Xue, P.; Zhao, J.; et al. Protecting Against Postsurgery Oral Cancer Recurrence with an Implantable Hydrogel Vaccine for In Situ Photoimmunotherapy. Adv. Sci. 2024, 11, 2309053. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Song, C.; Wei, Z.; Zou, H.; Han, S.; Cao, Z.; Zhang, X.; Zhang, G.; Ran, J.; Cai, Y.; et al. Multifunctional photodynamic/photothermal nano-agents for the treatment of oral leukoplakia. J. Nanobiotechnol. 2022, 20, 106. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Liu, T.; Li, Y.; Wan, C.; Gao, R.; Liu, C.; Li, X.; Li, B. Nano-drug delivery system targeting FAP for the combined treatment of oral leukoplakia. Drug Deliv. Transl. Res. 2023, 14, 247–265. [Google Scholar] [CrossRef]
- Zhu, T.; Sang, Z.; Ye, Z.; Guo, X.; Qu, X.; Hao, Y.; Wang, W. Local delivery of celecoxib/indocyanine green-loaded nanomodulators for combinational photothermal/photodynamic/anti-cyclooxygenase-2 therapy of oral leukoplakia. Chem. Eng. J. 2025, 505, 159734. [Google Scholar] [CrossRef]
- Shrivastava, R.; Dube, A. Effect of the polyelectrolyte coating on the photothermal efficiency of gold nanorods and the photothermal induced cancer cell damage. IET Nanobiotechnol. 2017, 11, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Nichols, J.W.; Toh, K.; Nomoto, T.; Cabral, H.; Miura, Y.; Christie, R.J.; Yamada, N.; Ogura, T.; Kano, M.R.; et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 2016, 11, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Shen, G.; Sun, J.; Abdelmohsen, L.K.E.A.; Yan, X.; van Hest, J.C.M. Flexible Morphological Regulation of Photothermal Nanodrugs: Understanding the Relationship between the Structure, Photothermal Effect, and Tumoral Biodistribution. ACS Nano 2025, 19, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, C.; Silva, W.F.; Garcia, J.; Zaragosa, G.P.; Ilem, C.N.D.; Sales, T.O.; Santos, H.D.A.; Conde, B.I.C.; Barbosa, H.P.; Malik, S.; et al. Nanoparticles based image-guided thermal therapy and temperature feedback. J. Mater. Chem. B 2025, 13, 54–102. [Google Scholar] [CrossRef]
- Xiao, Z.; Ji, C.; Shi, J.; Pridgen, E.M.; Frieder, J.; Wu, J.; Farokhzad, O.C. DNA self-assembly of targeted near-infrared-responsive gold nanoparticles for cancer thermo-chemotherapy. Angew. Chem.-Int. Edit. 2012, 51, 11853–11857. [Google Scholar] [CrossRef]
- Ren, Y.; Yan, Y.; Qi, H. Photothermal conversion and transfer in photothermal therapy: From macroscale to nanoscale. Adv. Colloid Interface Sci. 2022, 308, 102753. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Ganjali, F.; Zarei-Shokat, S.; Dinmohammadi, R.; Asl, F.R.; Emami, A.; Mojtabapour, Z.S.; Rashvandi, Z.; Kashtiaray, A.; Jalali, F.; et al. Plasmonic porous micro- and nano-materials based on Au/Ag nanostructures developed for photothermal cancer therapy: Challenges in clinicalization. Nanoscale Adv. 2023, 5, 6768–6786. [Google Scholar] [CrossRef]
- Nejabat, M.; Samie, A.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. An Overview on Gold Nanorods as Versatile Nanoparticles in Cancer Therapy. J. Control. Release 2023, 354, 221–242. [Google Scholar] [CrossRef]
- Alle, M.; Sharma, G.; Lee, S.-H.; Kim, J.-C. Next-generation engineered nanogold for multimodal cancer therapy and imaging: A clinical perspectives. J. Nanobiotechnol. 2022, 20, 222. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Li, Z.-Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef]
- Shekhar, S.; Chauhan, M.; Sonali; Yadav, B.; Dutt, R.; Hu, L.; Muthu, M.S.; Singh, R.P. Enhanced Permeability and Retention Effect-Focused Tumor-Targeted Nanomedicines: Latest Trends, Obstacles and Future Perspective. Nanomedicine 2022, 17, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, G.A.; Brandenburg, K.S.; Shakeri-Zadeh, A. A comparative study of two folate-conjugated gold nanoparticles for cancer nanotechnology applications. Cancers 2010, 2, 1911–1928. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Y.; Peng, X.; Li, W.; Yuan, Y.; Tao, X.; Yao, X.; Lv, R. Gold Nanostars Combined with the Searched Antibody for Targeted Oral Squamous Cell Carcinoma Therapy. ACS Biomater. Sci. Eng. 2022, 8, 2664–2675. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Wu, Y.; Tang, Y.; Xiao, H.; Chen, K.; Han, T.; Fang, N.; Wu, R.; El-Sayed, M.A. Targeting cancer cell integrins using gold nanorods in photothermal therapy inhibits migration through affecting cytoskeletal proteins. Proc. Natl. Acad. Sci. USA 2017, 114, E5655–E5663. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wu, J.; Jin, L.; Hong, L.; Wang, F.; Mao, Z.; Wu, M. Cancer cell membrane-coated gold nanorods for photothermal therapy and radiotherapy on oral squamous cancer. J. Mater. Chem. B 2020, 8, 7253–7263. [Google Scholar] [CrossRef]
- Ciftci, F.; Özarslan, A.C.; Kantarci, İ.C.; Yelkenci, A.; Tavukcuoglu, O.; Ghorbanpour, M. Advances in Drug Targeting, Drug Delivery, and Nanotechnology Applications: Therapeutic Significance in Cancer Treatment. Pharmaceutics 2025, 17, 121. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Shen, J.; Ding, D. Organic optical agents for image-guided combined cancer therapy. Biomed. Mater. 2021, 16, 042009. [Google Scholar] [CrossRef]
- Vyas, K.; Rathod, M.; Patel, M.M. Insight on nano drug delivery systems with targeted therapy in treatment of oral cancer. Nanomed. Nanotechnol. Biol. Med. 2023, 49, 102662. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, J.; Li, M.; Luo, M.; Dong, B.; Sitti, M.; Yan, X. NIR-II Fluorescent Thermophoretic Nanomotors for Superficial Tumor Photothermal Therapy. Adv. Mater. 2025, 37, 2417440. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R. Oral tumor heterogeneity, its implications for patient monitoring and designing anti-cancer strategies. Pathol. Res. Pract. 2024, 253, 154953. [Google Scholar] [CrossRef] [PubMed]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Mou, J.; Lin, T.; Huang, F.; Chen, H.; Shi, J. Black titania-based theranostic nanoplatform for single NIR laser induced dual-modal imaging-guided PTT/PDT. Biomaterials 2016, 84, 13–24. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Yang, G.; Wang, Y.; Ju, R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics 2021, 11, 6370–6392. [Google Scholar] [CrossRef]
- Juthani, R.; Punatar, S.; Mittra, I. New light on chemotherapy toxicity and its prevention. BJC Rep. 2024, 2, 41. [Google Scholar] [CrossRef]
- Aloss, K.; Hamar, P. Augmentation of the EPR effect by mild hyperthermia to improve nanoparticle delivery to the tumor. Biochim. Biophys. Acta-Rev. Cancer 2024, 1879, 189109. [Google Scholar] [CrossRef]
- Gormley, A.J.; Larson, N.; Sadekar, S.; Robinson, R.; Ray, A.; Ghandehari, H. Guided Delivery of Polymer Therapeutics Using Plasmonic Photothermal Therapy. Nano Today 2012, 7, 158–167. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Holtzman, A.L.; Dagan, R.; Bryant, C.M.; Hitchcock, K.E.; Amdur, R.J.; Fernandes, R.P. Current Role of Radiotherapy in the Management of Oral Cavity Squamous Cell Carcinoma. Craniomaxillofacial Trauma Reconstr. 2021, 14, 79–83. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Zhu, W.; Yi, X.; Dong, Z.; Xu, X.; Chen, M.; Yang, K.; Lu, G.; Jiang, L.; et al. Nanoscale metal-organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials 2016, 97, 1–9. [Google Scholar] [PubMed]
- Deng, Y.; Li, E.; Cheng, X.; Zhu, J.; Lu, S.; Ge, C.; Gu, H.; Pan, Y. Facile preparation of hybrid core-shell nanorods for photothermal and radiation combined therapy. Nanoscale 2016, 8, 3895–3899. [Google Scholar] [CrossRef]
- Guo, S.; Yao, Y.; Tang, Y.; Xin, Z.; Wu, D.; Ni, C.; Huang, J.; Wei, Q.; Zhang, T. Radiation-induced tumor immune microenvironments and potential targets for combination therapy. Signal Transduct. Target. Ther. 2023, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Lin, F. The Basic Properties of Gold Nanoparticles and their Applications in Tumor Diagnosis and Treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Teo, P.Y.; Cheng, W.; Hedrick, J.L.; Yang, Y.Y. Co-delivery of drugs and plasmid DNA for cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 41–63. [Google Scholar] [CrossRef]
- Tang, F.; Ding, A.; Xu, Y.; Ye, Y.; Li, L.; Xie, R.; Huang, W. Gene and Photothermal Combination Therapy: Principle, Materials, and Amplified Anticancer Intervention. Small 2024, 20, 2307078. [Google Scholar] [CrossRef]
- Li, M.; Hou, M.; Wu, Q.; Jiang, Y.; Jia, G.; Wu, X.; Zhang, C. Simultaneous Inhibition of Heat Shock Response and Autophagy with Bimetallic Mesoporous Nanoparticles to Enhance Mild-Temperature Photothermal Therapy. Small Struct. 2023, 4, 2300132. [Google Scholar] [CrossRef]
- Wang, S.; Tian, Y.; Tian, W.; Sun, J.; Zhao, S.; Liu, Y.; Wang, C.; Tang, Y.; Ma, X.; Teng, Z.; et al. Selectively Sensitizing Malignant Cells to Photothermal Therapy Using a CD44-Targeting Heat Shock Protein 72 Depletion Nanosystem. ACS Nano 2016, 10, 8578–8590. [Google Scholar] [CrossRef]
- Chang, M.; Hou, Z.; Wang, M.; Li, C.; Lin, J. Recent Advances in Hyperthermia Therapy-Based Synergistic Immunotherapy. Adv. Mater. 2021, 33, 2004788. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Goyal, P.M.; Kumar, M.; Kiran, M.; Srivastava, S.; Roy, S.K.; Garg, S.; Salunke, S.; Lader, S.; Quadri, K.; Ansari, A.; et al. Optimizing surgical margins in oral cancer without frozen section: A single center retrospective study. Eur. J. Surg. Oncol. 2025, 51, 109360. [Google Scholar] [CrossRef]
- Baddour, H.M., Jr.; Magliocca, K.R.; Chen, A.Y. The importance of margins in head and neck cancer. J. Surg. Oncol. 2016, 113, 248–255. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Migliacci, J.C.; Xu, B.; Katabi, N.; Montero, P.H.; Ganly, I.; Shah, J.P.; Wong, R.J.; Ghossein, R.A.; Patel, S.G. A Proposal to Redefine Close Surgical Margins in Squamous Cell Carcinoma of the Oral Tongue. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 555–560. [Google Scholar] [CrossRef]
- Smits, R.W.H.; Koljenović, S.; Hardillo, J.A.; Hove, I.T.; Meeuwis, C.A.; Sewnaik, A.; Dronkers, E.A.; Schut, T.C.B.; Langeveld, T.P.M.; Molenaar, J.; et al. Resection margins in oral cancer surgery: Room for improvement. Head Neck-J. Sci. Spec. Head Neck 2016, 38 (Suppl. S1), E2197–E2203. [Google Scholar]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008, 60, 977–985. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef]
- Yokoi, A.; Maruyama, T.; Yamanaka, R.; Takeuchi, N.; Morita, M.; Ekuni, D. Relationship among cancer treatment, quality of life, and oral function in head and neck cancer survivors: A cross-sectional study. Support. Care Cancer 2024, 32, 809. [Google Scholar] [CrossRef]
- Yuwanati, M.; Gondivkar, S.; Sarode, S.C.; Gadbail, A.; Desai, A.; Mhaske, S.; Pathak, S.K.; N Khatib, M. Oral Health-Related Quality of Life in Oral Cancer Patients: Systematic Review and Meta-Analysis. Future Oncol. 2021, 17, 979–990. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Montero, P.H.; Migliacci, J.C.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019, 90, 115–121. [Google Scholar] [CrossRef]
- Cortina, L.E.; Moverman, D.J.; Zhao, Y.; Goss, D.; Zenga, J.; Puram, S.V.; Varvares, M.A. Functional considerations between flap and non-flap reconstruction in oral tongue cancer: A systematic review. Oral Oncol. 2023, 147, 106596. [Google Scholar] [CrossRef]
- Al-Aroomi, M.A.; Al-Worafi, N.A.; Ma, Y.; Alkebsi, K.; Mohamed, A.A.S.; Jiang, C. Patient-reported outcomes after oral cancer reconstructions with radial and ulnar forearm-free flaps. Oral Dis. 2024, 30, 4878–4885. [Google Scholar] [CrossRef]
- Accorona, R.; Di Furia, D.; Cremasco, A.; Gazzini, L.; Mevio, N.; Pilolli, F.; Achena, A.; Iftikhar, H.; Awny, S.; Ormellese, G.L.; et al. Oral Reconstruction with Locoregional Flaps after Cancer Ablation: A Systematic Review of the Literature. J. Clin. Med. 2024, 13, 4181. [Google Scholar] [CrossRef]
- Nie, R.; Sun, Y.; Lv, H.; Lu, M.; Huangfu, H.; Li, Y.; Zhang, Y.; Wang, D.; Wang, L.; Zhou, Y. 3D printing of MXene composite hydrogel scaffolds for photothermal antibacterial activity and bone regeneration in infected bone defect models. Nanoscale 2022, 14, 8112–8129. [Google Scholar] [CrossRef]
- Wang, X.; Qian, Y.; Wang, S.; Wang, M.; Sun, K.; Cheng, Z.; Shao, Y.; Zhang, S.; Tang, C.; Chu, C.; et al. Accumulative Rolling Mg/PLLA Composite Membrane with Lamellar Heterostructure for Enhanced Bacteria Inhibition and Rapid Bone Regeneration. Small 2023, 19, 2301638. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Tan, B.; Shan, Y.; Zhao, X.; Liao, J. Near-infrared light control of GelMA/PMMA/PDA hydrogel with mild photothermal therapy for skull regeneration. Biomater. Adv. 2022, 133, 112641. [Google Scholar] [CrossRef]
- Liu, W.-S.; Chen, Z.; Lu, Z.-M.; Dong, J.-H.; Wu, J.-H.; Gao, J.; Deng, D.; Li, M. Multifunctional hydrogels based on photothermal therapy: A prospective platform for the postoperative management of melanoma. J. Control. Release 2024, 371, 406–428. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, H.; Ying, B.; Mei, X.; Zeng, D.; Liu, S.; Qu, W.; Pan, X.; Pu, S.; Li, R.; et al. Mild photothermal therapy assist in promoting bone repair: Related mechanism and materials. Mater. Today Bio 2023, 23, 100834. [Google Scholar] [CrossRef]
- Zhu, H.; Qian, C.; Xiao, W.; Zhang, Q.; Ge, Z. Performance of a porous composite scaffold containing silk fibroin: Applied research repair on oral jaw epithelial defects. Mater. Express 2020, 10, 490–502. [Google Scholar] [CrossRef]
- Gupta, P.; Adhikary, M.; Moses, J.C.; Kumar, M.; Bhardwaj, N.; Mandal, B.B. Biomimetic, Osteoconductive Non-mulberry Silk Fiber Reinforced Tricomposite Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 30797–30810. [Google Scholar] [CrossRef]
- Suzuki, A.; Kodama, Y.; Miwa, K.; Kishimoto, K.; Hoshikawa, E.; Haga, K.; Sato, T.; Mizuno, J.; Izumi, K. Manufacturing micropatterned collagen scaffolds with chemical-crosslinking for development of biomimetic tissue-engineered oral mucosa. Sci. Rep. 2020, 10, 22192. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mu, M.; Yan, J.; Han, B.; Ye, R.; Guo, G. 3D printing materials and 3D printed surgical devices in oral and maxillofacial surgery: Design, workflow and effectiveness. Regen. Biomater. 2024, 11, rbae066. [Google Scholar] [CrossRef] [PubMed]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Milan, E.P.; Martins, V.C.A.; Horn, M.M.; Plepis, A.M.G. Influence of blend ratio and mangosteen extract in chitosan/collagen gels and scaffolds: Rheological and release studies. Carbohydr Polym 2022, 292, 119647. [Google Scholar] [CrossRef]
- Loo, S.C.; Moore, T.; Banik, B.; Alexis, F. Biomedical applications of hydroxyapatite nanoparticles. Curr. Pharm. Biotechnol. 2010, 11, 333–342. [Google Scholar] [CrossRef]
- Dai, W.; Zheng, Y.; Li, B.; Yang, F.; Chen, W.; Li, Y.; Deng, Y.; Bai, D.; Shu, R. A 3D-printed orthopedic implant with dual-effect synergy based on MoS2 and hydroxyapatite nanoparticles for tumor therapy and bone regeneration. Colloids Surf. B-Biointerfaces 2023, 228, 113384. [Google Scholar] [CrossRef]
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care 2013, 2, 306–316. [Google Scholar] [CrossRef]
- Naara, S.; Andrews, C.; Sikora, A.; Williams, M.; Chambers, M.; Myers, J.; Amit, M. Oral Pre-malignancy: An Update on Novel Therapeutic Approaches. Curr. Oncol. Rep. 2024, 26, 1047–1056. [Google Scholar] [CrossRef]
- van der Waal, I. Historical perspective and nomenclature of potentially malignant or potentially premalignant oral epithelial lesions with emphasis on leukoplakia-some suggestions for modifications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 577–581. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Pimenta-Barros, L.A.; Ramos-García, P.; González-Moles, M.Á.; Aguirre-Urizar, J.M.; Warnakulasuriya, S. Malignant transformation of oral leukoplakia: Systematic review and comprehensive meta-analysis. Oral Dis. 2025, 31, 69–80. [Google Scholar] [CrossRef] [PubMed]
| Disease Type | Therapy Mode | Photothermal Agents | Functionalization | Exposure Conditions | Tumor Type | Therapeutic Highlights | Ref. |
|---|---|---|---|---|---|---|---|
| Oral Cancer | PTT | AuNRs | PEG conjugated | 808 nm, 5.8 W cm−2 | HSC 3 cells | 28 × 8 nm AuNRs are a more effective photothermal contrast agent for PTT of OSCC | [59] |
| Thiolated PEG conjugated | 808 nm, 0.9–1.9 W cm−2 | HSC 3 cells, CDX mouse model | Preferential tumor accumulation of Pegylated AuNRs indicates the selectivity and specificity of PTT | [60] | |||
| Folate conjugated | 755 nm, 40 J cm−2 | KB cells | Folic acid for active tumor targeting | [61] | |||
| Cysteine-functionalized alginate and cyclic peptide, c(RGDfK)KKK modified | 808 nm, 2 W cm−2 | SAS 3 cells, CDX mouse models | Replacing cetyltrimethylammonium bromide (CTAB) with alginate improves the biocompatibility of AuNRs | [62] | |||
| PLTs loaded | 808 nm, 2 W cm−2 | CAL 27 cells, CDX mouse model, HNSCC-bearing Tgfbr1/Pten 2cKO mouse model | PTT enhances tumor targeting of PLT-AuNRs, which in turn improves PTT effects via a feedback mechanism, demonstrating the benefit of PLT-PTT in cancer therapy | [63] | |||
| Anti-EGFR antibodies conjugated | 800 nm, 10–20 W cm−2 | HOC 313 cells, HSC 3 cells | Integrated molecular imaging with photothermal cancer therapy | [64] | |||
| AuNPs | — | 532 nm, 0.3 W cm−2 | DMBA-induced HBP carcinoma | Plasmonic photothermal therapy on induced HBP carcinoma | [65] | ||
| Anti-EGFR antibodies conjugated | 514 nm, 13–64 W cm−2 | HSC 313 cells, HOC 3 cells | Selective PTT for epithelial carcinoma | [66] | |||
| CuS NPs | BSA-templated synthesis of BSA@CuS nanoparticles with subsequent PEGylation | 1064 nm, 0.5 W cm−2 | CAL 27 cells, SCC 9 cells, CDX mouse model | Modified with PEG to increase biocompatibility | [67] | ||
| Ag3AuS2 NPs | Ag3AuS2 NPs complexed with genetically engineered anionic protein and chitosan | 808 nm, 1 W cm−2 | CAL 27 cells, CDX mouse model | Features tongue tumor inhibition and complication prevention | [68] | ||
| Gold-silica nanoshells | Anti-HER2 nanobodies conjugated | 820 nm, 4 W cm−2 | KB cells | First in vitro investigation of PTT efficacy using anti-HER2 nanobody-conjugated nanoshells in an OSCC model | [69] | ||
| Fe3O4 NPs | Platelet-cancer stem cell hybrid membrane coated | 808 nm, 5 W cm−2 | CAL 27 cells, CDX mouse model, HNSCC-bearing Tgfbr1/Pten 2cKO mouse model | First presentation of a platelet–cancer stem cell hybrid membrane-coated iron oxide magnetic nanoparticle for enhanced PTT of HNSCC | [70] | ||
| Au@C NPs | Membrane of patient-derived cells coated | 808 nm, 1 W cm−2 | CAL 27 cells, SCC 7 cells, HN 6 cells, CDX mouse model, orthotopic tongue tumor mouse model, primary and distant tumor mouse models, PDX mouse model | The homologous cancer cell membrane provided the nanoplatforms with optimal targeting properties for maximum therapeutic efficiency | [71] | ||
| BP NSs | PDA and polyacrylamide hydrochloride-dimethylmaleic acid (PAH-DMMA) charge reversal system modified | 808 nm, 1.5 W cm−2 | CAL 27 cells, SAS cells, CDX mouse model | Nanoplatforms exhibit suitable size for intravenous delivery, enrichment in tumor sites, enhanced tumor cell uptake, excellent photothermal properties, and effective oral cancer cell killing | [72] | ||
| Carbon dots | Triton-X-directed synthesis of N-rich mesoporous carbon nanospheres from pyrrole and aniline | 980 nm, 1.4 W cm−2 | FaDu cells | Exhibiting integrated PTT and FL functionalities | [73] | ||
| Semiconductor polymer (PCPDTBT) | Incorporated gadolinium-grafted triblock amphiphilic copolymer (F127-DTPA-Gd) | 808 nm, 1 W cm−2 | SCC 7 cells, CDX mouse model | Two-component nanotheranostic platform enabling efficient MRI and FL-guided PTT | [74] | ||
| GO | Amino-modified | 808 nm, 2 W cm−2 | HSC 3 cells, CDX mouse model | Graphene-based nanomaterials as direct nano-PTAs for anticancer PTT | [50] | ||
| Gold nanodots | Peptide HN-1 modified | 808 nm, 2 W cm−2 | SCC 9 cells, CAL 27 cells, CRL 1623 cells, CDX mouse model | Versatile nanosystem for targeted drug delivery and diagnostic imaging | [54] | ||
| Au/Mn nanodots | — | 1064 nm, 2 W cm−2 | SCC 9 cells, CAL 27 cells, CDX mouse model | Features multimodal bioimaging, including concurrent CT and MRI, and bright near-infrared FL for navigation | [75] | ||
| ICG | Functionalized with cypate fluorophore and two cyclic-(arginine-glycine-aspartic acid) (cRGD) peptides | 808 nm, 1 W cm−2 | CAL 27 cells, CDX mouse model | ICG-derived NIR fluorescent probes designed and synthesized for accurate diagnosis and treatment | [76] | ||
| Aggregation-induced emission luminogens (AIEgen) | NMB@NPs constructed from carbon radical monomer, ethyl 2,6-diisocyanatohexanoate, PEG molecules, and an AIEgen | 808 nm, 1 W cm−2 | CAL 27 cells, PDX mouse model | FL-guided thermodynamic therapy and PTT | [77] | ||
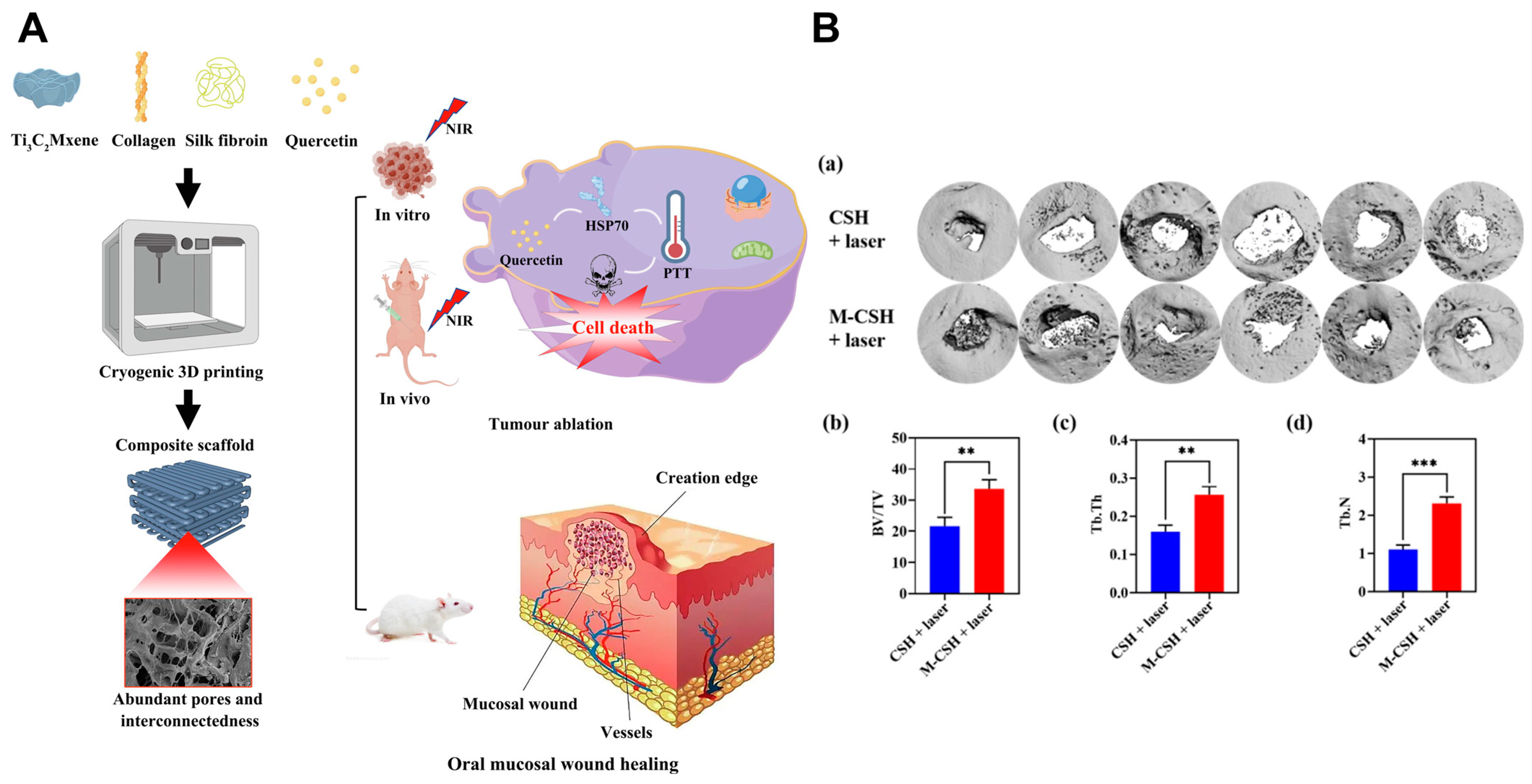
| MXene NSs | Incorporated into a scaffold created from collagen, silk, and hydroxyapatite | 808 nm, 1 W cm−2 | CAL 27 cells, bone defect rabbit model | Simultaneously kills OSCC cells and promotes bone tissue regeneration | [56] | ||
| Ti3C2 Mxene | Scaffold constructed from Ti3C2 MXene, collagen, and silk fibroin | 808 nm, 1 W cm−2 | SCC 25 cells, CAL 27 cells, CDX mouse model | Exhibits simultaneous OSCC cell cytotoxicity and mucosal defect regeneration | [57] | ||
| ICG | Scaffold fabricated with collagen/silk fibroin and ICG | 808 nm, 1 W cm−2 | SCC 25 cells, CDX mouse model | Facilitated the attachment and proliferation of rat buccal mucosa fibroblasts and enhanced the repair of buccal mucosal wounds | [58] | ||
| PTT, PDT | AuNRs | Rose bengal molecules conjugated | 810 nm, 17.86 W cm−2, 532 nm, 1.76 W cm−2 | CAL 27 cells, DMBA-induced HBP carcinoma | Combined PDT/PTT capabilities against oral cancer | [78] | |
| Organic compound (C3) | ICG and C3 encapsulated within PEG-PCL | 808 nm, 0.5 W cm−2 | HSC cells, CDX mouse model | FL-guided PTT/PDT against OSCC | [45] | ||
| Au nanoflower | Two layers of silica shell ICG added | 808 nm, 10 W cm−2 | Cal 27 cells, CDX mouse model | Tumor growth inhibition through synchronous PTT and PDT | [79] | ||
| AuNPs | Sulfonated aluminum phthalocyanines conjugated | 1064 nm, 39.9–420.1 W cm−2 | SAS cells | Effective inactivation of oral cancer cells via combined PTT and PDT effects | [80] | ||
| Au nanopopcorns | Stabilized with PEG through 11-mercaptoundecanoic acid and coated with silicon 2,3-naphthalocyanine dihydroxide | 808 nm, 0.55 W cm−2 | KB-3-1 cells, SK-BR-3 cells | First application of magnetic-field-guided drug delivery and dual-mode PTT/PDT using magnetic-optical hybrid nanosystems | [81] | ||
| Cu2−xS | Magnetic manganese compounds integrated | 808 nm, 0.72 W cm−2 | HeLa cells, CDX mouse model, PDX mouse model | Reactive oxygen species (ROS) and heat generation enhance PTT, and O2 self-supplementation enhances PDT | [82] | ||
| ICG, SWCNTs | ICG-conjugated hyaluronic acid nanoparticles encapsulated within SWCNTs | 808 nm, 0.8 W cm−2 | SCC 7 cells, CDX mouse model | CD44-targeted theranostic nanoparticle for PA image-guided dual PTT and PDT cancer therapy | [83] | ||
| Cobalt-glycerate nanosheets | Folic acid modified | 808 nm, 1.2 W cm−2 | CAL 27 cells, residual OSCC tumors bearing mouse model, CDX mouse model | MRI-guided postsurgical PTT/PDT | [84] | ||
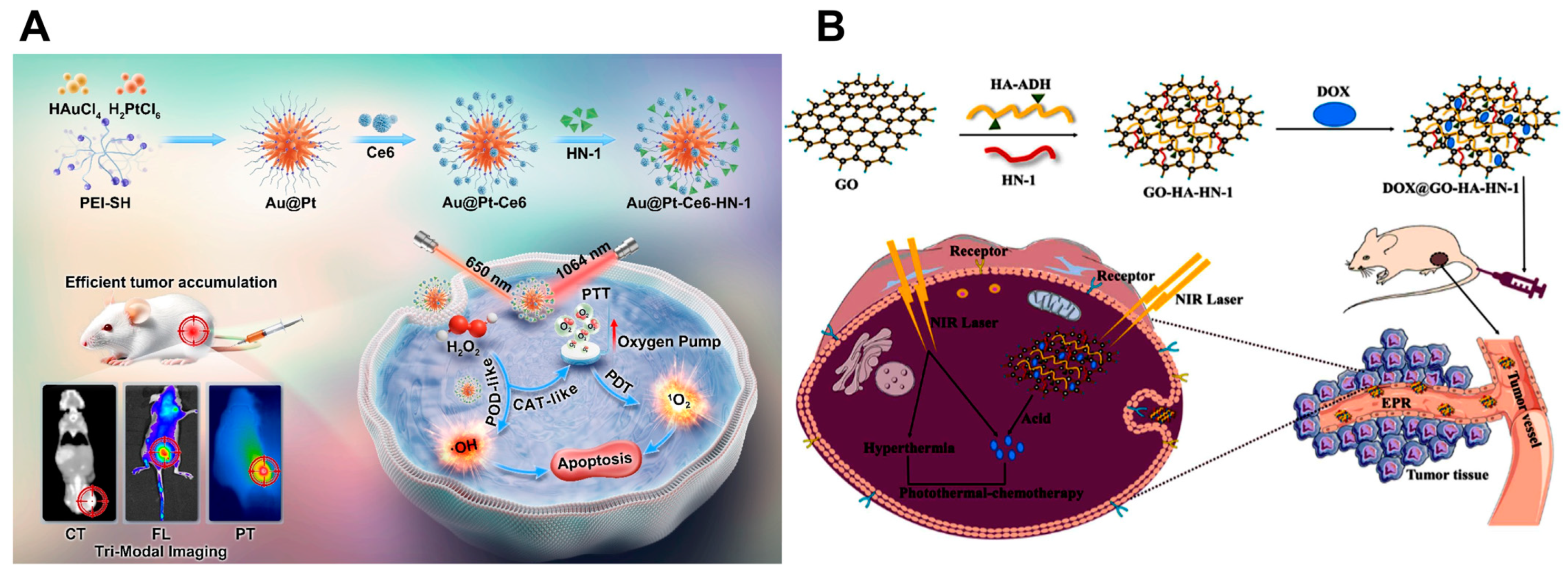
| Au, Ce6 | Polyethyleneimine functionalized with Au and Pt, followed by attachment of Ce6 and HN-1 | 1064 nm, 2 W cm−2, 650 nm, 0.5 W cm−2 | SCC 9 cells, CDX mouse model | CT/FL/photothermal tri-modal imaging-guided treatment | [85] | ||
| PTT, chemotherapy | ICG | Nanoparticles co-assembled from hydrophilic linear PEG and hydrophobic cholic acid cluster amphiphilic subunits | 808 nm, 0.8 W cm−2 | OSC 3 cells, orthotopic CDX mouse model, metastatic CDX mouse model | Versatile chemo-nanoplatform for synergistic PTT/chemotherapy of orthotopic oral cancer and immuno-nanoplatform for synergistic PTT/immunotherapy of metastatic cancer | [86] | |
| DOX-encapsulated PLGA nanoparticles with a cancer cell membrane and ICG surface coating | 808 nm, 1.5 W cm−2 | HSC 3 cells, CDX mouse model | Selective cancer cell targeting and induction of intrinsic mitochondria-mediated apoptosis via the p53 signaling pathway | [55] | |||
| IR820 | IR820/methylcellulose hydrogel containing mesoporous silica nanoparticles and DOX | 808 nm, 2 W cm−2 | CAL 27 cells, CDX mouse model | Long-term synergistic antitumor activity with lower toxicity | [87] | ||
| IR820 and curcumin loaded onto hyaluronic acid microneedles | 808 nm, 1 W cm−2 | CAL 27 cells | Curcumin nanoparticles and IR820 microneedle combined drug delivery systems (DDS) have complete morphology and good mechanical properties | [88] | |||
| AuNRs | Silica-coated AuNRs with a covalently assembled amphiphilic PLGA-PEG polymeric corona loaded with vincristine | 808 nm, 1.2 W cm−2 | SCC 15 cells, DMBA-induced HBP carcinoma | Coronabased drug delivery approach exhibited superior anticancer effects on OSCC | [89] | ||
| Folate-targeted pegylated poly(D, L-lactide-co-glycolide) loaded with phytochemical anticancer thymoquinone and AuNRs | 808 nm, 1.2 W cm−2 | SCC 15 cells, DMBA-induced HBP carcinoma | Strong synergistic anticancer effects and selective tumor targeting via a dual-modal approach | [90] | |||
| AuNPs | Endogenously double-controlled cisplatin prodrug incorporated | 808 nm, 0.3 W cm−2 | 3D tumor models of SCC-25 and SCC-154 | The first multifunctional nano-architecture (tNAscisPt) for combined chemotherapy and PTT | [91] | ||
| PEG-stabilized and conjugated with PDPN antibody and DOX | 532 nm, 1 W cm−2 | CAL 27 cells, CDX mouse model | Versatile drug-delivery nanoplatforms for targeted and combined chemo-PTT against oral cancer | [92] | |||
| GO | PEGylated GO linked with DOX and FAP-targeted peptide chains | 808 nm, 1 W cm−2 | CAL 27 cells, CDX mouse model | Precise targeting capability coupled with combined chemotherapy and PTT | [93] | ||
| Modified with hyaluronic acid and HN-1 peptide and loaded with DOX | 808 nm, 1 W cm−2 | CAL 27 cells, CDX mouse model | Effective targeting of OSCC cells and outstanding localized deposition in xenograft tumors | [94] | |||
| Loaded with ATP citrate lyase specific inhibitor (SB-204990) and DOX | 808 nm, 1.5 W cm−2 | SCC 15 cells, CDX mouse model | Synergistic treatment via lipid starvation, chemotherapy, and PTT | [95] | |||
| GO and Cisplatin blended with poly(L-lactide) and hydroxypropyl methylcellulose | 808 nm, 1.5 W cm−2 | Human oral squamous cell carcinoma cell line UPCI-SCC-084, cisplatin-resistant cell line, 3D tumor spheroid model | 3D-printed biodegradable implant with chemo-thermal ablation potential | [96] | |||
| Cu (II) | Enveloped by chitosan | 808 nm, 0.33 W cm−2 | KB cells, CDX mouse model | Combined PTT and chemotherapy using CuCC NPs for noninvasive tumor ablation and reduced postoperative recurrence risk | [97] | ||
| Polyethylene glycol-coated polyaniline NSs codoped with Cu (II) and vincristine | 808 nm, 0.33 W cm−2 | KBV cells, KB cells, CDX mouse model | MDR-tumor-targeted theranostics utilizing strong electrostatic interactions between resistant cells and nanomaterials | [98] | |||
| Au nanoflowers | Two layers of silica coated | 808 nm, 5–9 W cm−2 | CAL 27 cells, HepG2 cells | Potentials for versatile loading and delivery of chemotherapeutic or photodynamic drugs | [99] | ||
| Hollow mesoporous Prussian blue NPs (HMPBs) | DOX-loaded HMPBs within a hyaluronic acid microneedle system | 808 nm, 2 W cm−2 | CAL 27 cells, CDX mouse model | Thermal ablation and DOX release, promoted by the generated heat, induced apoptosis of tumor cells | [100] | ||
| Chiral molybdenum (Cys-MoO3−x) NPs | Decorated with cysteine molecules | 405–808 nm, 1 W cm−2 | OSCC cells | Visible light and NIR dual-responsive properties for cancer cell ablation | [101] | ||
| PDA | Functionalized with S-nitrosothiol, surrounded by gambogic acid-conjugated hyaluronic acid shells | 808 nm, 1 W cm−2 | HN6 cells, CDX mouse model | Tumor-selective nanocomplex for low-temperature photothermal therapy and NO-enhanced chemotherapy | [102] | ||
| PTT, radiotherapy | AuNPs | Folate conjugated. | 532 nm, 0.47 W cm−2 | KB cells | Folate-conjugated gold nanoparticles induced cytotoxicity and cell apoptosis in KB cells | [103] | |
| PTT, gene therapy | AuNRs | Complexed with anionic-charged siRNA oligos | 810 nm, 3.3 W cm−2 | CAL 27 cells, CDX mouse model | Overcomes thermoresistance to sensitize cancer cells to hyperthermia | [104] | |
| PTT, immunotherapy | ICG | Gelatin nanoparticles loaded with ICG and NSC74859 (signal transducer activator of transcription 3, STAT3 inhibitor) | 808 nm, 1 W cm−2 | CAL 27 cells, CDX mouse model, HNSCC-bearing Tgfbr1/Pten 2cKO mouse model | Photothermal destruction of tumors combined with the STAT3 inhibitor elicited potent antitumor immunity for enhanced cancer therapy | [105] | |
| Organic photovoltaic material (PBDB-T NPs) | — | 660 nm, 0.6 W cm−2 | CAL 27 cells, CDX mouse model, immunocompetent and syngeneic mouse tumor model | Enhanced mPTT efficacy and active tumor-specific adaptive immune responses | [106] | ||
| Molybdenum diphosphide nanorods (MoP2 NRs) | — | 808 nm, 0.5 W cm−2 | CAL 27 cells, SCC9 cells, CDX mouse model | Therapeutic modality via laser-potentiated peroxidase catalytic/mPTT | [107] | ||
| Fe3O4 NPs | — | 808 nm, 1 W cm−2 | SCC 7 cells, CDX mouse model | Regulates the polarization of tumor-associated macrophages and enhances the inhibitory effect on tumor cells | [108] | ||
| IR820 | TAT peptide-conjugated IR820 incorporated into Poly(N-isopropylacrylamide) (PNIPAM)/demethylated lignin (DL) hydrogel | 808 nm, 2 W cm−2 | SCC 7 cells, bacteria-colonized tumor spheroids, bacteria-colonized tumor-bearing mouse, lung metastasis model with bacterial colonization | Photothermal ablation with robust stimulation of antitumor immune responses against bacteria-colonized OSCC | [109] | ||
| PTT, PDT, chemotherapy | AuNPs | Combined with cisplatin-loaded BP NSs | 808 nm | SCC 9 cells, DMBA-induced HBP carcinoma | High drug-loading capacity and excellent photothermal properties | [110] | |
| ICG | Coordination compound of ICG-cisplatin encapsulated into human serum albumin | 808 nm, 2 W cm−2 | HSC cells, CDX mouse model | Synergistic PTT/PDT/chemotherapy against OSCC via a NIR stimuli-responsive, tumor-targeted drug release system | [111] | ||
| Coi8DFIC-sorafenib NPs | Coi8DFIC dye and sorafenib were used to construct CS NPs | 808 nm, 0.5 W cm−2 | CAL 27 cells, CDX mouse model | Laser on/off controlled vascular targeting therapy with CS NPs, guided by tumor-associated vessel tracking and real-time tumor imaging | [112] | ||
| Pheophorbidea, Pa | Pa and DOX self-assembled, followed by the introduction of dual-aldehyde terminated polyethylene glycol2000 (PEG-2CHO) | 680 nm, 0.4 W cm−2 | OSC 3 cells, subcutaneous and orthotopic CDX tumor model | Dual size/charge-transformable Trojan-Horse nanoparticle for delivery of ultrasmall, full active pharmaceutical ingredients | [113] | ||
| PTT, PDT, gene therapy | ICG | Poly(β-amino ester)/PLGA blended nanoparticles loaded with ICG, surface-adsorbed with Nrf2-siRNA, and encapsulated within cancer cell membrane vesicles | 808 nm, 2 W cm−2 | SCC 25 cells, CDX mouse model | Excellent PTT/PDT agent for OSCC treatment, where Nrf2-siRNA serves as an efficient photosensitizer synergist for PDT amplification | [114] | |
| PTT, PDT, immunotherapy | Ce6 | Simvastatin (SIM)-packaged Ce6-PEG with a surface modification of targeting-antibody (anti-low-density lipoprotein receptor) | 660 nm, 1 W cm−2 | SCC 7 cells, homologous xenograft tumor mouse model | Cholesterol-regulating NPs with high tumor targeting and adjuvanticity for effective photo-induced immunotherapy | [115] | |
| IR780 | Double-layered membrane vesicles extracted from attenuated P. gingivalis as an immune adjuvant | 808 nm, 1 W cm−2 | SCC 7 cells, CDX mouse model, metastatic CDX mouse model | Double-layered membrane vesicles derived from P. gingivalis applied as a novel bacterial adjuvant for activating antitumor immunity | [116] | ||
| PDA | PDA-hyaluronic acid matrix loaded with protoporphyrin IX and aCD47-tagged CaCO3 NPs | 808 nm, 1.2 W cm−2, 660 nm, 0.04 W cm−2 | CAL 33 cells, low-immunogenic OSCC mouse model, residual tumor mouse model, orthotopic and subcutaneous CDX mouse model | Effectively prevents local recurrence, inhibits orthotopic OSCC growth and pulmonary metastasis, and provides long-term protective immunity against tumor rechallenge | [117] | ||
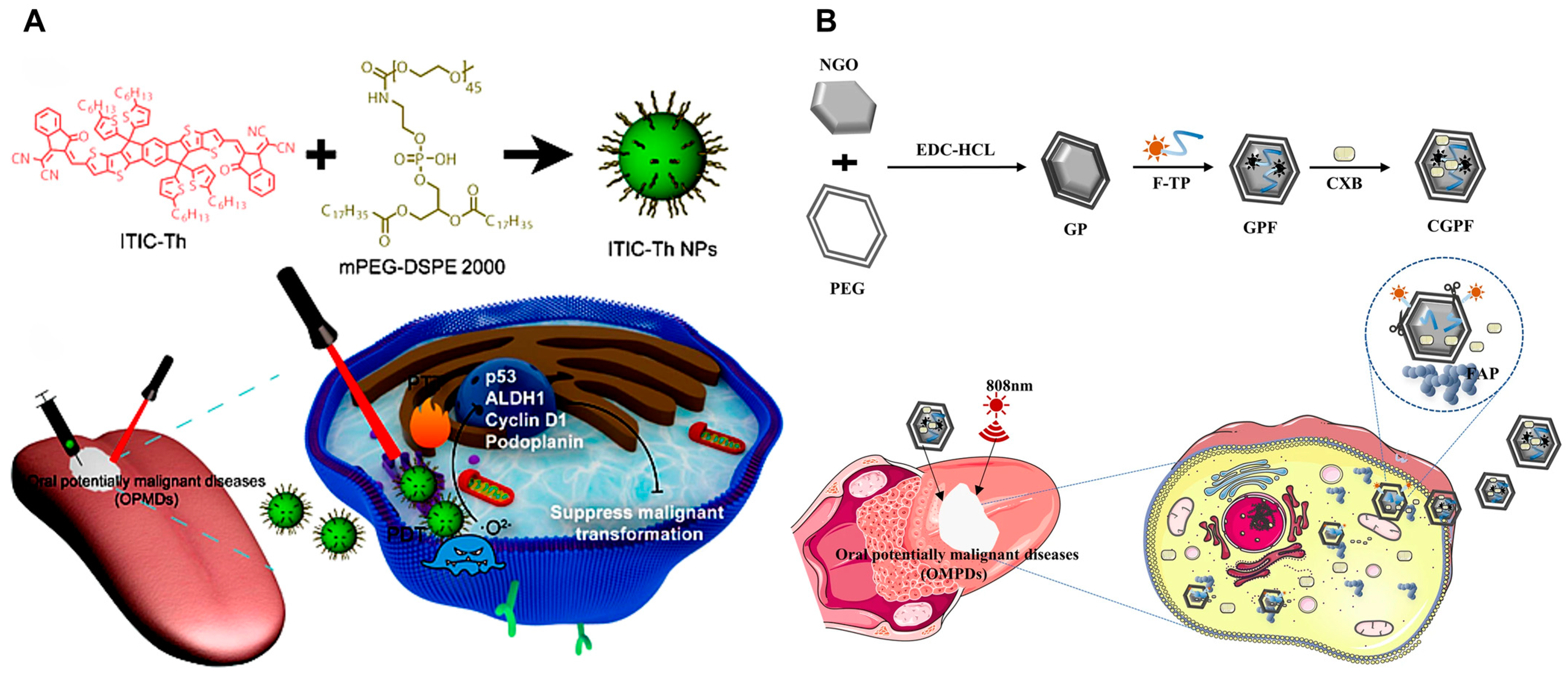
| Oral leu-koplakia (OLK) | PTT, PDT | ITIC-Th | — | 660 nm, 1 W cm−2 | CAL 27 cells, 4-nitroquinoline 1-oxide (4NQO)-induce oral leukoplakia mouse model | Effectively suppresses OLK cancerization without apparent topical or systemic toxicity and represents the first interdisciplinary research in multimodal therapy for OLK | [118] |
| PTT, drug therapy | GO | Surface modification of GO with PEG via amide reaction, serving as a carrier to adsorb FAP targeting peptides and cyclooxygenase-2 inhibitors | 808 nm, 1 W cm−2 | DOK cells, 4-nitroquinoline 1-oxide (4NQO)-induced oral precancerous mice model | Nano-drug delivery system for targeting OLK with high FAP expression | [119] | |
| PTT, PDT, drug therapy | Mesoporous polydopamine nanoparticles | ICG and celecoxib co-loaded onto mesoporous polydopamine nanoparticles | 808 nm, 1.5 W cm−2 | DOK cells, 4-nitroquinoline 1-oxide (4NQO)-induced oral precancerous mice model | Transmucosal delivery of soluble microneedle-mediated integrated phototherapy anti-inflammatory NPs for OLK | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Wang, P.; Lin, Y.; Jia, A.; Tong, F.; Li, Z. Advances in Photothermal Therapy for Oral Cancer. Int. J. Mol. Sci. 2025, 26, 4344. https://doi.org/10.3390/ijms26094344
Liang J, Wang P, Lin Y, Jia A, Tong F, Li Z. Advances in Photothermal Therapy for Oral Cancer. International Journal of Molecular Sciences. 2025; 26(9):4344. https://doi.org/10.3390/ijms26094344
Chicago/Turabian StyleLiang, Jian, Pei Wang, Yanfang Lin, Ao Jia, Fei Tong, and Zhihua Li. 2025. "Advances in Photothermal Therapy for Oral Cancer" International Journal of Molecular Sciences 26, no. 9: 4344. https://doi.org/10.3390/ijms26094344
APA StyleLiang, J., Wang, P., Lin, Y., Jia, A., Tong, F., & Li, Z. (2025). Advances in Photothermal Therapy for Oral Cancer. International Journal of Molecular Sciences, 26(9), 4344. https://doi.org/10.3390/ijms26094344






