Vitamins in the Pathogenesis of Prostate Cancer: Implications for Prevention and Therapeutic Support
Abstract
1. Introduction
2. Methods
3. Role of Fat-Soluble Vitamins in Prostate Cancer
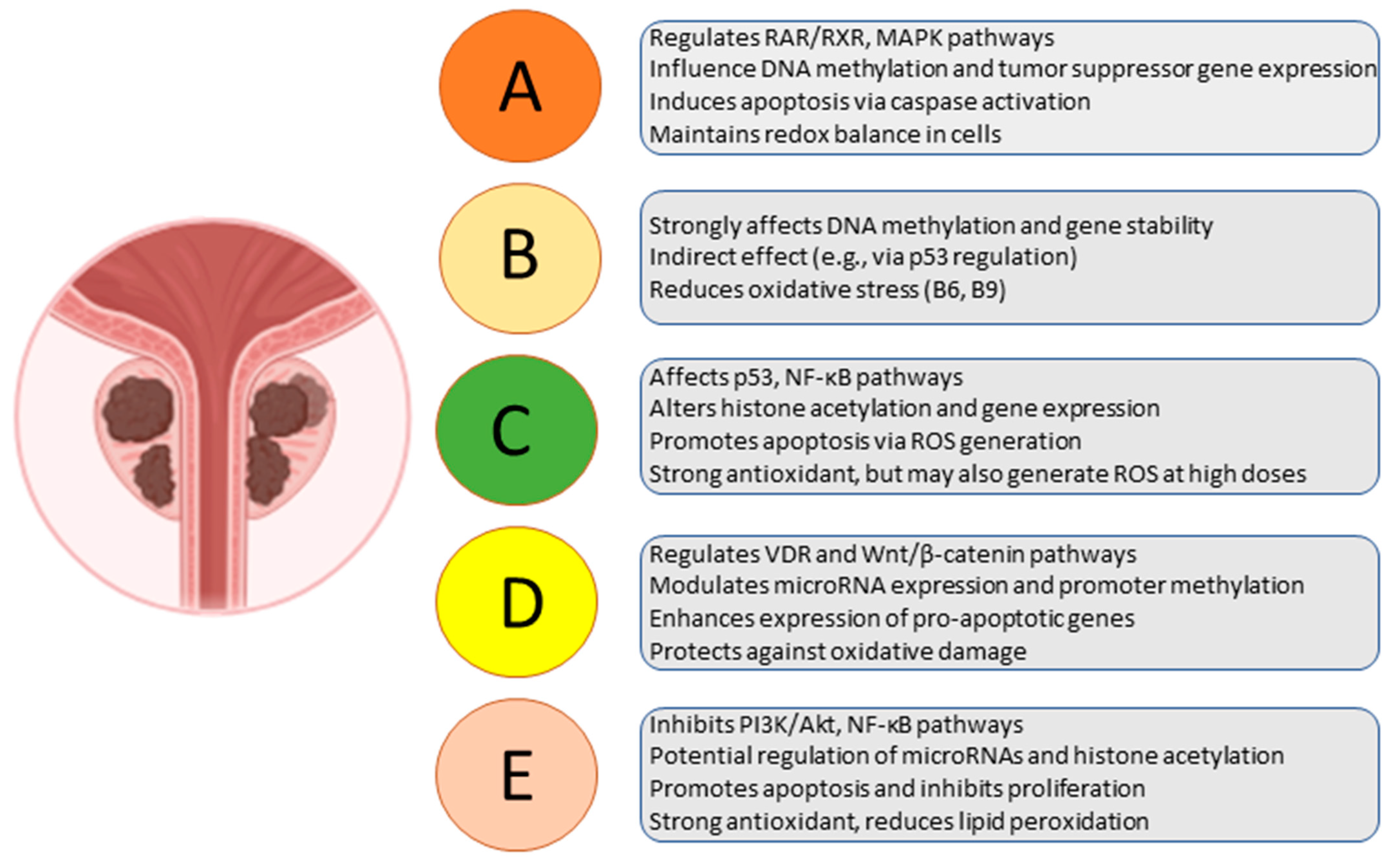
3.1. Vitamin D
3.2. Vitamin A and Derivatives
3.3. Vitamin E
4. Role of Water-Soluble Vitamins in Prostate Cancer
4.1. Vitamin B Group
4.2. Vitamin C
5. Role of Vitamins in Prostate Cancer Prevention
6. Vitamins as Support for Prostate Cancer Therapy
7. Balancing Benefits and Risks of Vitamins Intake in Prostate Cancer Prevention and Therapy
8. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schafer, E.J.; Laversanne, M.; Sung, H.; Soerjomataram, I.; Briganti, A.; Dahut, W.; Bray, F.; Jemal, A. Recent Patterns and Trends in Global Prostate Cancer Incidence and Mortality: An Update. Eur. Urol. 2025, 87, 302–313. [Google Scholar] [CrossRef]
- Cao, W.; Qin, K.; Li, F.; Chen, W. Comparative study of cancer profiles between 2020 and 2022 using global cancer statistics (GLOBOCAN). J. Natl. Cancer Cent. 2024, 4, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory-Europe; Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; et al. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 24 April 2025).
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; Vecchia, C.L. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef]
- Adeloye, D.; David, R.A.; Aderemi, A.V.; Iseolorunkanmi, A.; Oyedokun, A.; Iweala, E.E.J.; Omoregbe, N.; Ayo, C.K. An Estimate of the Incidence of Prostate Cancer in Africa: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0153496. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Men’s Health. 2019, 37, 288–295. [Google Scholar] [CrossRef]
- Nouri-Majd, S.; Salari-Moghaddam, A.; Aminianfar, A.; Larijani, B.; Esmaillzadeh, A. Association Between Red and Processed Meat Consumption and Risk of Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 801722. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef]
- PDQ Integrative A and Complementary Therapies Editorial Board. Prostate Cancer, Nutrition, and Dietary Supplements (PDQ®): Health Professional Version; National Cancer Institute (US): Bethesda, MD, USA, 2002.
- Datta, M.; Schwartz, G.G. Calcium and vitamin D supplementation during androgen deprivation therapy for prostate cancer: A critical review. Oncologist 2012, 17, 1171–1179. [Google Scholar] [CrossRef]
- Nair-Shalliker, V.; Smith, D.P.; Gebski, V.; Patel, M.I.; Frydenberg, M.; Yaxley, J.W.; Gardiner, R.; Espinoza, D.; Kimlin, M.G.; Fenech, M.; et al. High-dose vitamin D supplementation to prevent prostate cancer progression in localised cases with low-to-intermediate risk of progression on active surveillance (ProsD): Protocol of a phase II randomised controlled trial. BMJ Open 2021, 11, e044055. [Google Scholar] [CrossRef]
- Russnes, K.M.; Möller, E.; Wilson, K.M.; Carlsen, M.; Blomhoff, R.; Smeland, S.; Adami, H.-O.; Grönberg, H.; Mucci, L.A.; Bälter, K. Total antioxidant intake and prostate cancer in the Cancer of the Prostate in Sweden (CAPS) study. A case control study. BMC Cancer 2016, 16, 438. [Google Scholar] [CrossRef]
- Stevens, S.L. Fat-Soluble Vitamins. Nurs. Clin. N. Am. 2021, 56, 33–45. [Google Scholar] [CrossRef]
- Bruno, E.J.; Ziegenfuss, T.N. Water-soluble vitamins: Research update. Curr. Sports Med. Rep. 2005, 4, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Rago, V.; Agostino, S.D. Novel Insights into the Role of the Antioxidants in Prostate Pathology. Antioxidants 2023, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Hsing, A.W.; Reichardt, J.K.; Stanczyk, F.Z. Hormones and prostate cancer: Current perspectives and future directions. Prostate 2002, 52, 213–235. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Physiology, Molecular Biology, and Clinical Applications; Humana Press: Totowa, NJ, USA, 2010. [Google Scholar]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Chen, T.C.; Holick, M.F. Vitamin D and prostate cancer prevention and treatment. Trends Endocrinol. Metab. 2003, 14, 423–430. [Google Scholar] [CrossRef]
- Skowronski, R.J.; Peehl, D.M.; Feldman, D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology 1993, 132, 1952–1960. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Mantell, D.J.; Owens, P.E.; Bundred, N.J.; Mawer, E.B.; Canfield, A.E. 1α,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000, 87, 214–220. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Bao, B.-Y.; Yeh, S.-D.; Lee, Y.-F. 1α,25-dihydroxyvitamin D3 inhibits prostate cancer cell invasion via modulation of selective proteases. Carcinogenesis 2006, 27, 32–42. [Google Scholar] [CrossRef]
- Travis, R.C.; Crowe, F.L.; Allen, N.E.; Appleby, P.N.; Roddam, A.W.; Tjonneland, A.; Olsen, A.; Linseisen, J.; Kaaks, R.; Boeing, H.; et al. Serum vitamin D and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition (EPIC). Am. J. Epidemiol. 2009, 169, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Batai, K.; Kittles, R.A. Can vitamin D supplementation reduce prostate cancer disparities? Pharmacogenomics 2016, 17, 1117–1120. [Google Scholar] [CrossRef]
- Gilbert, R.; Martin, R.M.; Beynon, R.; Harris, R.; Savovic, J.; Zuccolo, L.; Bekkering, G.E.; Fraser, W.D.; Sterne, J.A.C.; Metcalfe, C. Associations of circulating and dietary vitamin D with prostate cancer risk: A systematic review and dose–response meta-analysis. Cancer Causes Control. 2011, 22, 319–340. [Google Scholar] [CrossRef]
- Trump, D.L.; Potter, D.M.; Muindi, J.; Brufsky, A.; Johnson, C.S. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer 2006, 106, 2136–2142. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am. J. Clin. Nutr. 2007, 85, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996, 10, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.H.; Gudas, L.J. Retinoids, retinoic acid receptors, and cancer. Annu. Rev. Pathol. 2011, 6, 345–364. [Google Scholar] [CrossRef]
- Niles, R.M. Signaling pathways in retinoid chemoprevention and treatment of cancer. Mutat. Res. 2004, 555, 81–96. [Google Scholar] [CrossRef]
- Jin, Y.; Teh, S.S.; Lau, H.L.N.; Xiao, J.; Mah, S.H. Retinoids as anti-cancer agents and their mechanisms of action. Am. J. Cancer Res. 2022, 12, 938–960. [Google Scholar]
- Key, T.J.; Appleby, P.N.; Allen, N.E.; Travis, R.C.; Roddam, A.W.; Jenab, M.; Egevad, L.; Tjønneland, A.; Johnsen, N.F.; Overvad, K.; et al. Plasma carotenoids, retinol, and tocopherols and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition study. Am. J. Clin. Nutr. 2007, 86, 672–681. [Google Scholar] [CrossRef]
- Donkena, K.V.; Karnes, R.J.; Young, C.Y.F. Vitamins and prostate cancer risk. Molecules 2010, 15, 1762–1783. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Weinstein, S.J.; Albanes, D. Vitamins, metabolomics, and prostate cancer. World J. Urol. 2017, 35, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free. Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Es-Sai, B.; Wahnou, H.; Benayad, S.; Rabbaa, S.; Laaziouez, Y.; El Kebbaj, R.; Limami, Y.; Duval, R.E. Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Molecules 2025, 30, 653. [Google Scholar] [CrossRef]
- Jiang, Q.; Im, S.; Wagner, J.G.; Hernandez, M.L.; Peden, D.B. Gamma-tocopherol, a major form of vitamin E in diets: Insights into antioxidant and anti-inflammatory effects, mechanisms, and roles in disease management. Free. Radic. Biol. Med. 2022, 178, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef]
- Ho, E.; Beaver, L.M.; Williams, D.E.; Dashwood, R.H. Dietary Factors and Epigenetic Regulation for Prostate Cancer Prevention. Adv. Nutr. 2011, 2, 497–510. [Google Scholar] [CrossRef]
- Collin, S.M.; Metcalfe, C.; Refsum, H.; Lewis, S.J.; Zuccolo, L.; Smith, G.D.; Chen, L.; Harris, R.; Davis, M.; Marsden, G.; et al. Circulating folate, vitamin B12, homocysteine, vitamin B12 transport proteins, and risk of prostate cancer: A case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomark. Prev. 2010, 19, 1632–1642. [Google Scholar] [CrossRef]
- Lin, P.-H.; Aronson, W.; Freedland, S.J. Nutrition, dietary interventions and prostate cancer: The latest evidence. BMC Med. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Bai, X.-Y.; Qu, X.; Jiang, X.; Xu, Z.; Yang, Y.; Su, Q.; Wang, M.; Wu, H. Association between Dietary Vitamin C Intake and Risk of Prostate Cancer: A Meta-analysis Involving 103,658 Subjects. J. Cancer 2015, 6, 913–921. [Google Scholar] [CrossRef]
- Parent, M.-E.; Richard, H.; Rousseau, M.-C.; Trudeau, K. Vitamin C Intake and Risk of Prostate Cancer: The Montreal PROtEuS Study. Front. Physiol. 2018, 9, 1218. [Google Scholar] [CrossRef] [PubMed]
- Trump, D.L.; Deeb, K.K.; Johnson, C.S. Vitamin D: Considerations in the continued development as an agent for cancer prevention and therapy. Cancer J. 2010, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Vitamin D supplements and cancer incidence and mortality: A meta-analysis. Br. J. Cancer 2014, 111, 976–980. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.; Tangen, C.M.; Lucia, M.S.; Goodman, P.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; Karp, D.D.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). J. Clin. Oncol. 2011, 30, 7. [Google Scholar] [CrossRef]
- Block, G. Vitamin C and cancer prevention: The epidemiologic evidence. Am. J. Clin. Nutr. 1991, 53 (Suppl. 1), 270S–282S. [Google Scholar] [CrossRef]
- Nimptsch, K.; Rohrmann, S.; Linseisen, J. Dietary intake of vitamin K and risk of prostate cancer in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg). Am. J. Clin. Nutr. 2008, 87, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.H.; Kristal, A.R.; Stanford, J.L. Fruit and vegetable intakes and prostate cancer risk. J. Natl. Cancer Inst. 2000, 92, 61–68. [Google Scholar] [CrossRef]
- Giovannucci, E. Nutritional factors in human cancers. Adv. Exp. Med. Biol. 1999, 472, 29–42. [Google Scholar]
- Godoy, A.S.; Chung, I.; Montecinos, V.P.; Buttyan, R.; Johnson, C.S.; Smith, G.J. Role of androgen and vitamin D receptors in endothelial cells from benign and malignant human prostate. Am. J. Physiol. Metab. 2013, 304, E1131–E1139. [Google Scholar] [CrossRef][Green Version]
- Trump, D.L.; Aragon-Ching, J.B. Vitamin D in prostate cancer. Asian J. Androl. 2018, 20, 244–252. [Google Scholar] [CrossRef]
- Klimant, E.; Wright, H.; Rubin, D.; Seely, D.; Markman, M. Intravenous vitamin C in the supportive care of cancer patients: A review and rational approach. Curr. Oncol. 2018, 25, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Moritz, B.; Schmitz, A.E.; Rodrigues, A.L.S.; Dafre, A.L.; Cunha, M.P. The role of vitamin C in stress-related disorders. J. Nutr. Biochem. 2020, 85, 108459. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, J.; Messing, E.M.; Chang, E.; Yang, C.-R.; Yeh, S. Vitamin E succinate inhibits the function of androgen receptor and the expression of prostate-specific antigen in prostate cancer cells. Proc. Natl. Acad. Sci. USA 2002, 99, 7408–7413. [Google Scholar] [CrossRef]
- Cardenas, E.; Ghosh, R. Vitamin E: A dark horse at the crossroad of cancer management. Biochem. Pharmacol. 2013, 86, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. Gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar] [CrossRef]
- Garland, C.F.; French, C.B.; Baggerly, L.L.; Heaney, R.P. Vitamin D supplement doses and serum 25-hydroxyvitamin D in the range associated with cancer prevention. Anticancer. Res. 2011, 31, 607–611. [Google Scholar]
- Brändstedt, J.; Almquist, M.; Manjer, J.; Malm, J. Vitamin D, PTH, and calcium and the risk of prostate cancer: A prospective nested case-control study. Cancer Causes Control 2012, 23, 1377–1385. [Google Scholar] [CrossRef]
- Vieth, R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am. J. Clin. Nutr. 1999, 69, 842–856. [Google Scholar] [CrossRef]
- Price, A.J.; Travis, R.C.; Appleby, P.N.; Albanes, D.; Gurrea, A.B.; Bjørge, T.; Bueno-De-Mesquita, H.B.; Chen, C.; Donovan, J.; Gislefoss, R.; et al. Circulating Folate and Vitamin B12 and Risk of Prostate Cancer: A Collaborative Analysis of Individual Participant Data from Six Cohorts Including 6875 Cases and 8104 Controls. Eur. Urol. 2016, 70, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.D.K.; Elinder, C.-G.; Tiselius, H.-G.; Wolk, A.; Akesson, A. Ascorbic acid supplements and kidney stone incidence among men: A prospective study. JAMA Intern. Med. 2013, 173, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.; Flower, G.; Weeks, L.; Cooley, K.; Callachan, M.; McGowan, J.; Skidmore, B.; Kirchner, L.; Seely, D. Intravenous Vitamin C and Cancer: A Systematic Review. Integr. Cancer Ther. 2014, 13, 280–300. [Google Scholar] [CrossRef]
- Nash, S.H.; Till, C.; Song, X.; Lucia, M.S.; Parnes, H.L.; Thompson, I.M.; Lippman, S.M.; Platz, E.A.; Schenk, J. Serum Retinol and Carotenoid Concentrations and Prostate Cancer Risk: Results from the Prostate Cancer Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Watters, J.L.; Männistö, S.; Weinstein, S.J.; Snyder, K.; Virtamo, J.; Albanes, D. Serum retinol and risk of prostate cancer. Am. J. Epidemiol. 2011, 173, 813–821. [Google Scholar] [CrossRef]

| Vitamin | Solu-bility | Storage Location | Role in the Body | Role in Cancer | Ref. |
|---|---|---|---|---|---|
| A (Retinol) | Fat | Liver, adipose tissue | Vision, immunity, skin and mucous membrane health | May have antineoplastic effects by regulating cell proliferation and differentiation, but excess intake may promote liver cancer | [13] |
| D (Cholecalciferol, Ergocalciferol) | Fat | Liver, adipose tissue | Regulation of calcium-phosphorus balance, bone health | May reduce the risk of certain cancers (e.g., colorectal) by regulating the cell cycle and inducing apoptosis | [10,11] |
| E (Tocopherol) | Fat | Adipose tissue, adrenal glands | Antioxidant, protects cell membranes | May have protective effects against cancer as an antioxidant, but high doses may increase the risk of prostate cancer | [13,15] |
| K (Phylloquinone, Menaquinone) | Fat | Liver | Blood clotting, bone metabolism | May have carcinopreventive effects by inhibiting cancer cell proliferation | [13] |
| B Vitamins (B1, B2, B3, B5, B6, B7, B9, B12) | Water | No significant location, small amounts in the liver and muscles | Metabolism of carbohydrates, lipids, and proteins, DNA synthesis, nervous and hematopoietic system function | May have protective effects against some cancers (e.g., colorectal, breast, esophageal), but excess intake of certain B vitamins (e.g., B9, B12) may promote cancer development and progression | [14] |
| C (Ascorbic Acid) | Water | No significant location | Antioxidant, collagen synthesis, immune function | May have protective effects as an antioxidant, but high doses may support the growth of certain cancers under specific conditions | [12,14] |
| Characteristic | Alpha-Tocopherol (α-T) | Gamma-Tocopherol (γ-T) |
|---|---|---|
| Level in the body | Predominates in human serum due to selective transport in the liver | Present in smaller amounts but also detectable in serum |
| Antioxidant activity | Strong antioxidant that protects cell membranes from oxidative stress | Potentially more effective in protecting against peroxynitrite-induced lipid oxidation |
| Impact on prostate cancer | Conflicting study results—supplementation in the SELECT trial increased prostate cancer risk | Potentially protective effect—inverse correlation with the risk of advanced prostate cancer |
| Anticarcinogenic mechanisms | Protects cells from oxidative stress but may disrupt the balance of other tocopherols | Induces apoptosis, inhibits cancer cell proliferation, and activates tumor-suppressing receptors (e.g., PPAR-γ) |
| Effect on tocopherol balance | High doses may reduce γ-T levels | Naturally present in the diet without negatively affecting α-T levels |
| Main Mechanism of Action | Impact on Prostate Cancer | Role in Therapy | Concerns and Limitations | Positive Impact (dose) | Ref. | |
|---|---|---|---|---|---|---|
| Vit. A | Regulation of proliferation, differentiation, and apoptosis through activation of RAR and RXR receptors | Inhibition of cancer cell proliferation and invasiveness, regulation of the cell cycle | May increase cell sensitivity to hormonal therapy and chemotherapy | Clinical trial results are inconclusive; further research is needed on safety | 2500–5000 IU/day (from diet, e.g., beta-carotene) | [31,32] |
| Vit. B | Involvement in DNA synthesis, epigenetic regulation, transport via haptocorrin and transcobalamin | Conflicting results—low levels may increase risk, while supplementation may promote tumor progression | Impact on DNA methylation, which may modulate oncogene expression | Supplementation may increase cancer progression risk, while dietary folates may have protective effects | 2.4 µg/day (from diet or moderate supplementation) | [64] |
| Vit. C | Antioxidant properties, reduction of oxidative stress and inflammation | May improve patients’ quality of life and support therapy | May increase cell sensitivity to hormonal therapy and chemotherapy | High doses may exhibit pro-oxidant effects and influence chemotherapy effectiveness | 75–200 mg/day (from diet) | [45] |
| Vit. D | Regulation of cell proliferation and differentiation via activation of the VDR receptor | May slow cancer cell proliferation and enhance therapy effectiveness | May increase cell sensitivity to hormonal therapy and chemotherapy | No conclusive clinical evidence on supplementation efficacy; further studies are needed | 800–2000 IU/day (maintenance of 25(OH)D: 50–125 nmol/L) | [46] |
| Vit. E | Modulation of androgen receptor levels, impact on cancer cell growth | May inhibit prostate cancer cell proliferation and reduce androgen receptor activity | May increase cell sensitivity to hormonal therapy and chemotherapy | Conflicting study results; in the SELECT trial, α-tocopherol supplementation increased prostate cancer risk | <100 IU/day (from diet, gamma-tocopherol) | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Królikowska, K.; Kiślak, J.; Orywal, K.; Zajkowska, M. Vitamins in the Pathogenesis of Prostate Cancer: Implications for Prevention and Therapeutic Support. Int. J. Mol. Sci. 2025, 26, 4336. https://doi.org/10.3390/ijms26094336
Królikowska K, Kiślak J, Orywal K, Zajkowska M. Vitamins in the Pathogenesis of Prostate Cancer: Implications for Prevention and Therapeutic Support. International Journal of Molecular Sciences. 2025; 26(9):4336. https://doi.org/10.3390/ijms26094336
Chicago/Turabian StyleKrólikowska, Kinga, Jakub Kiślak, Karolina Orywal, and Monika Zajkowska. 2025. "Vitamins in the Pathogenesis of Prostate Cancer: Implications for Prevention and Therapeutic Support" International Journal of Molecular Sciences 26, no. 9: 4336. https://doi.org/10.3390/ijms26094336
APA StyleKrólikowska, K., Kiślak, J., Orywal, K., & Zajkowska, M. (2025). Vitamins in the Pathogenesis of Prostate Cancer: Implications for Prevention and Therapeutic Support. International Journal of Molecular Sciences, 26(9), 4336. https://doi.org/10.3390/ijms26094336








