Novel Tear Biomarkers in Ocular Graft Versus Host Disease Associated with Th1/Th2 Immune Responses: A Case Series and Literature Review
Abstract
1. Introduction
2. Results
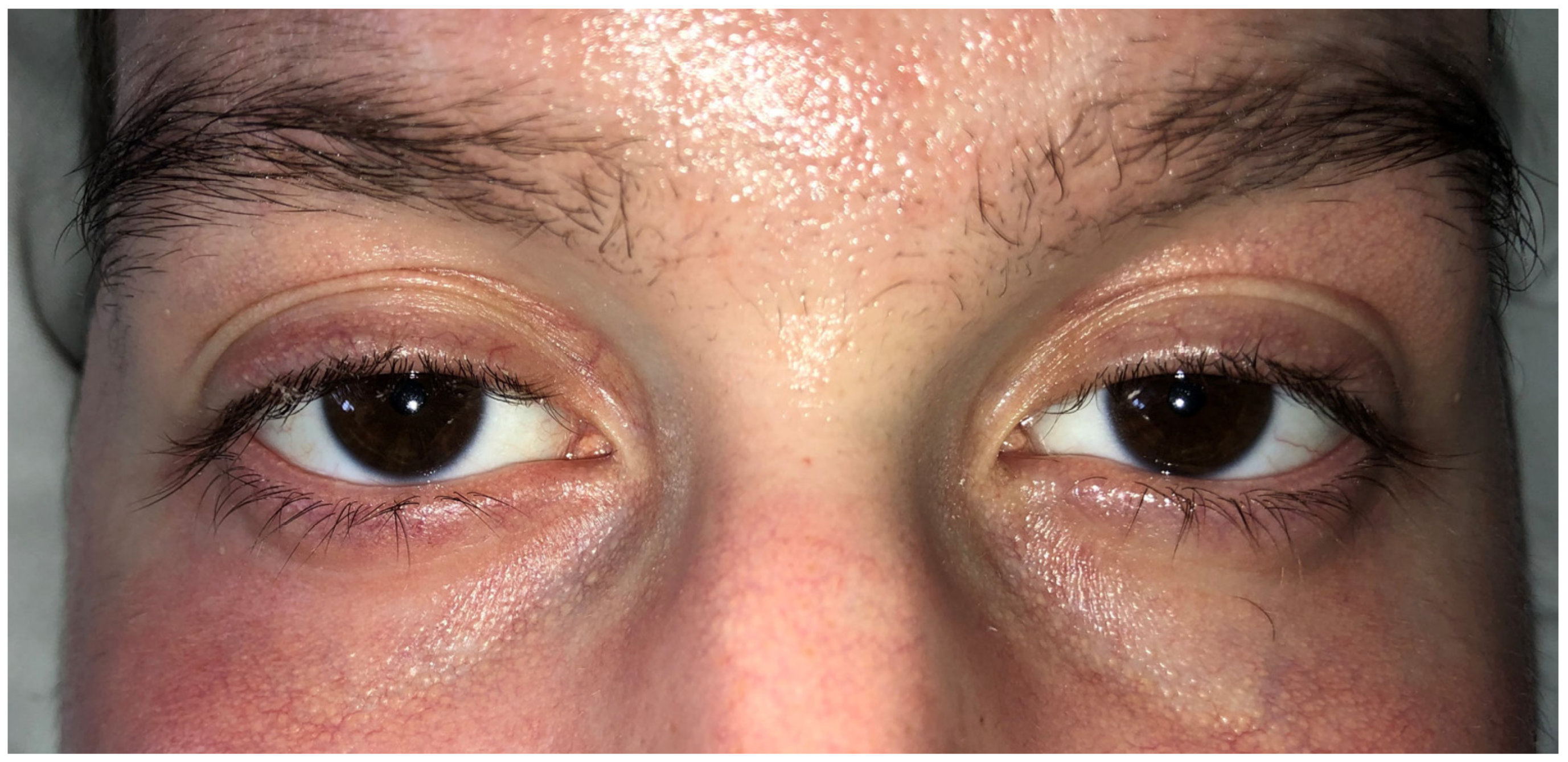
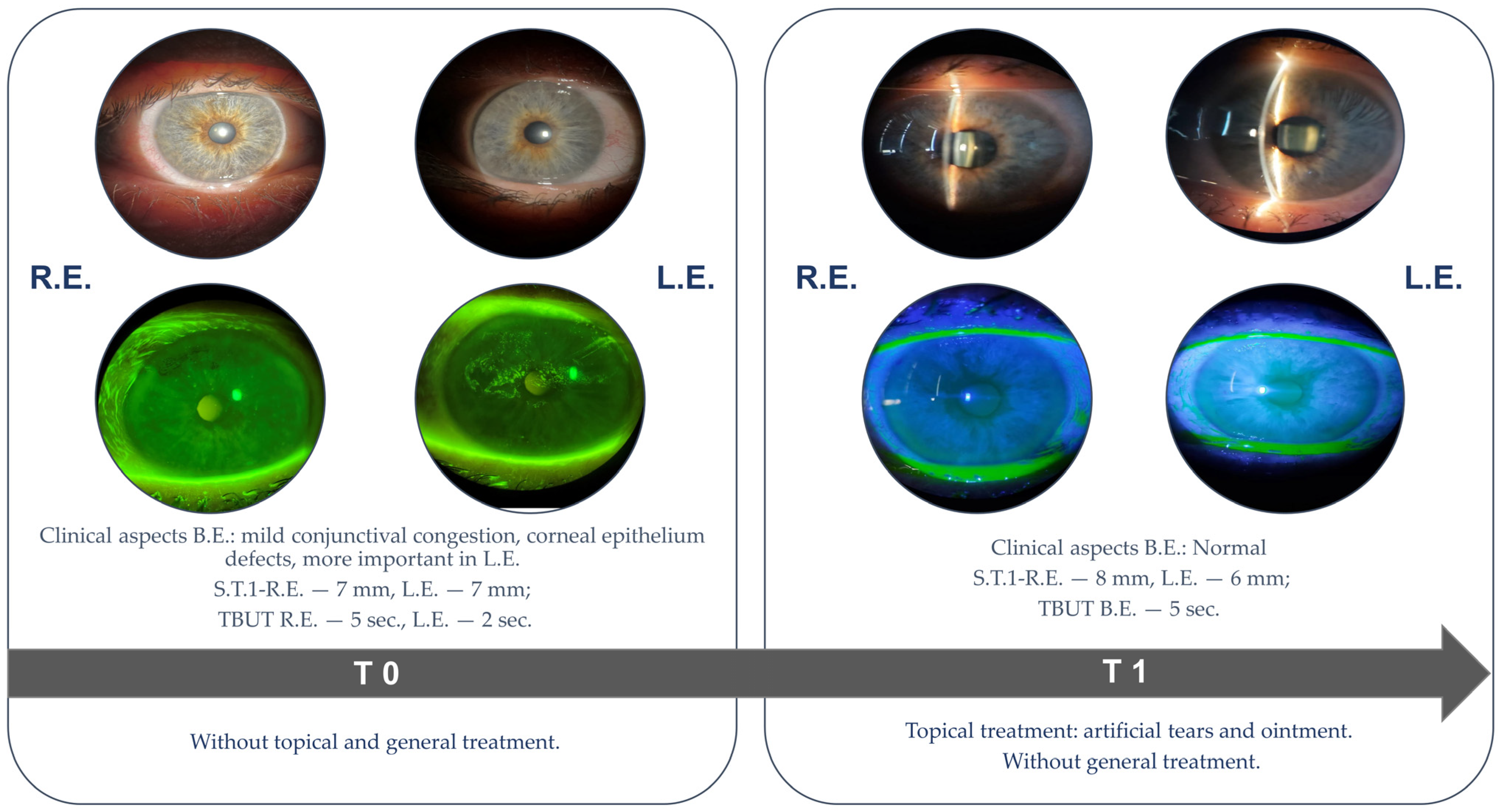
2.1. Case Presentation
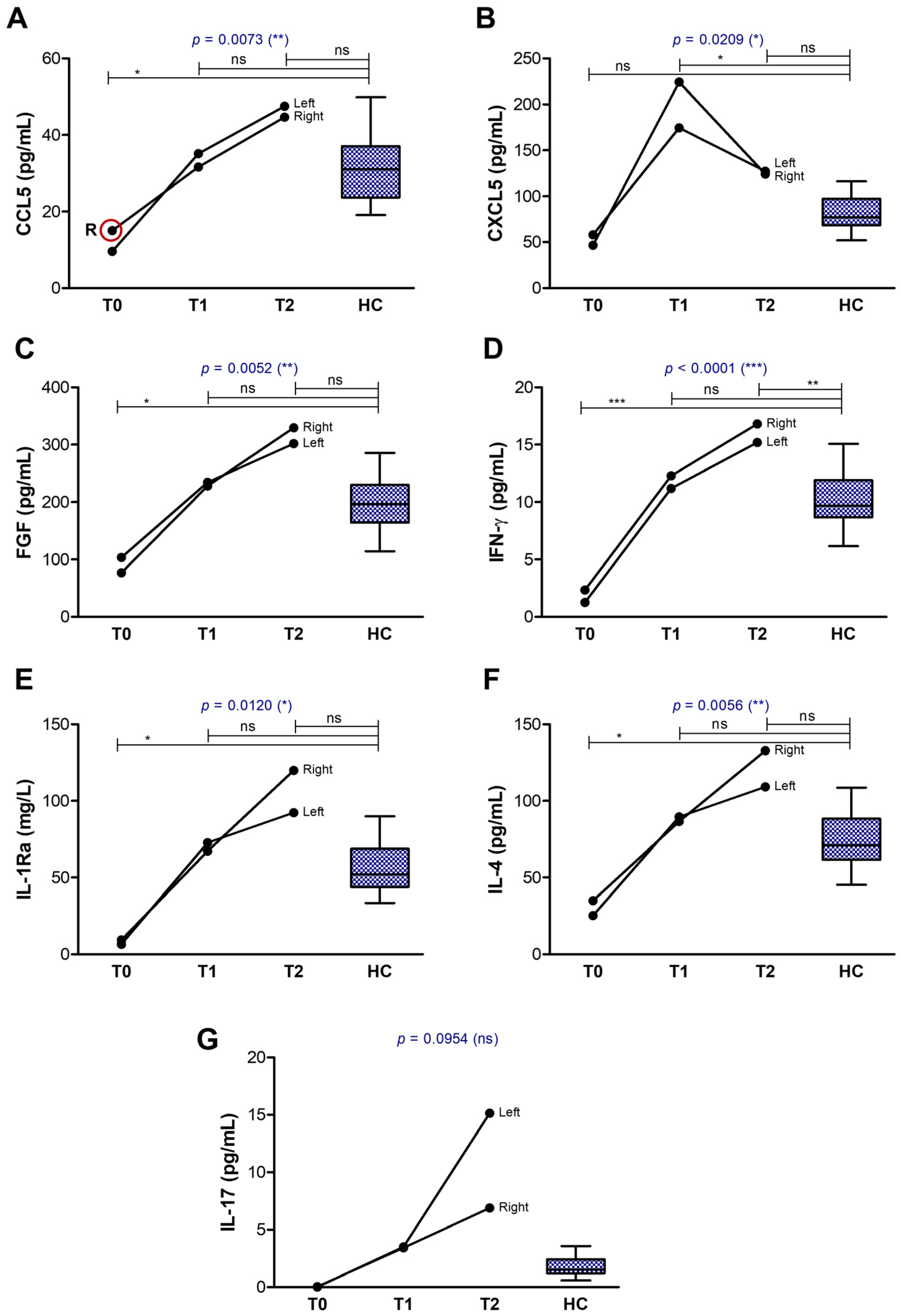
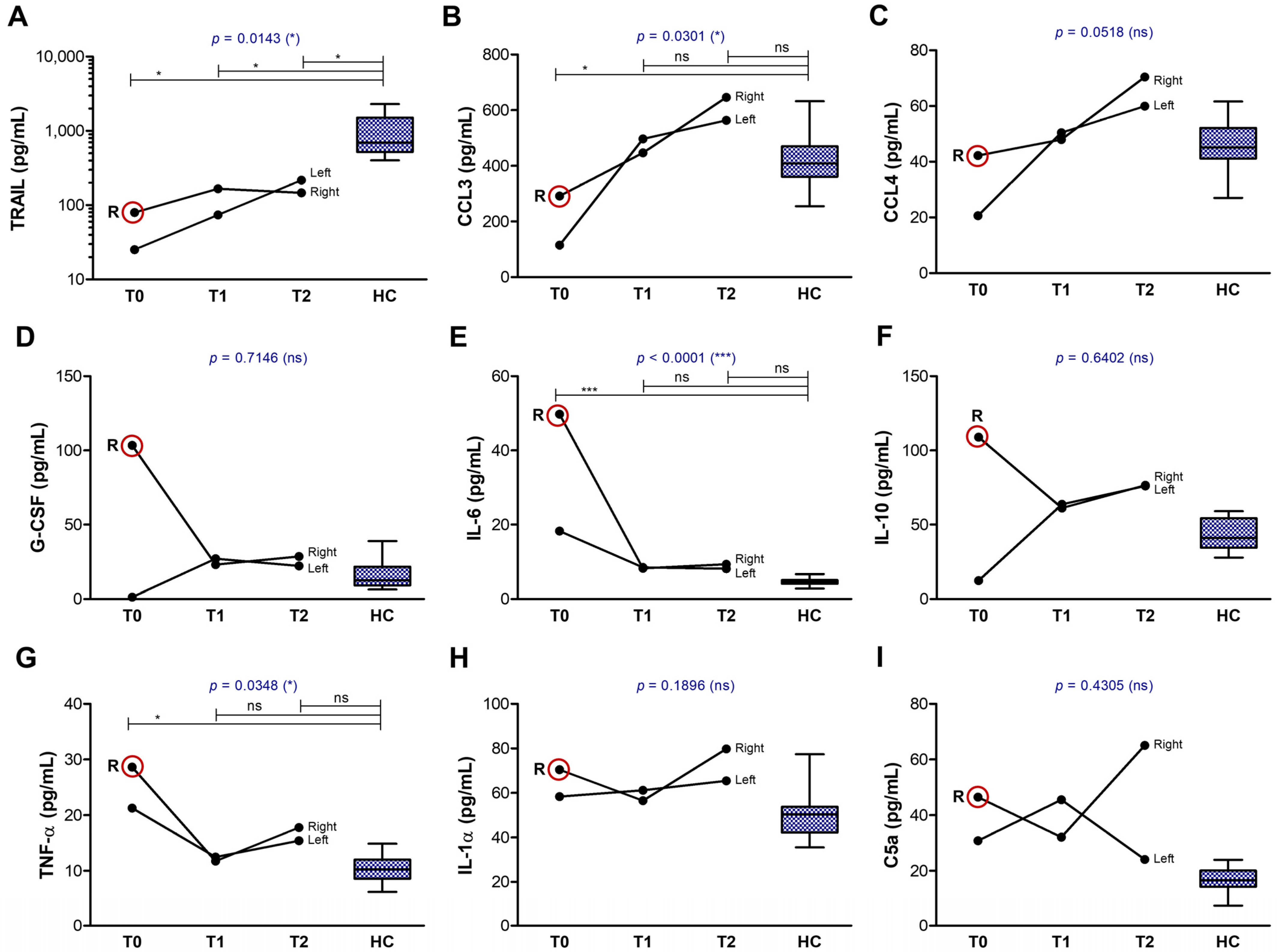
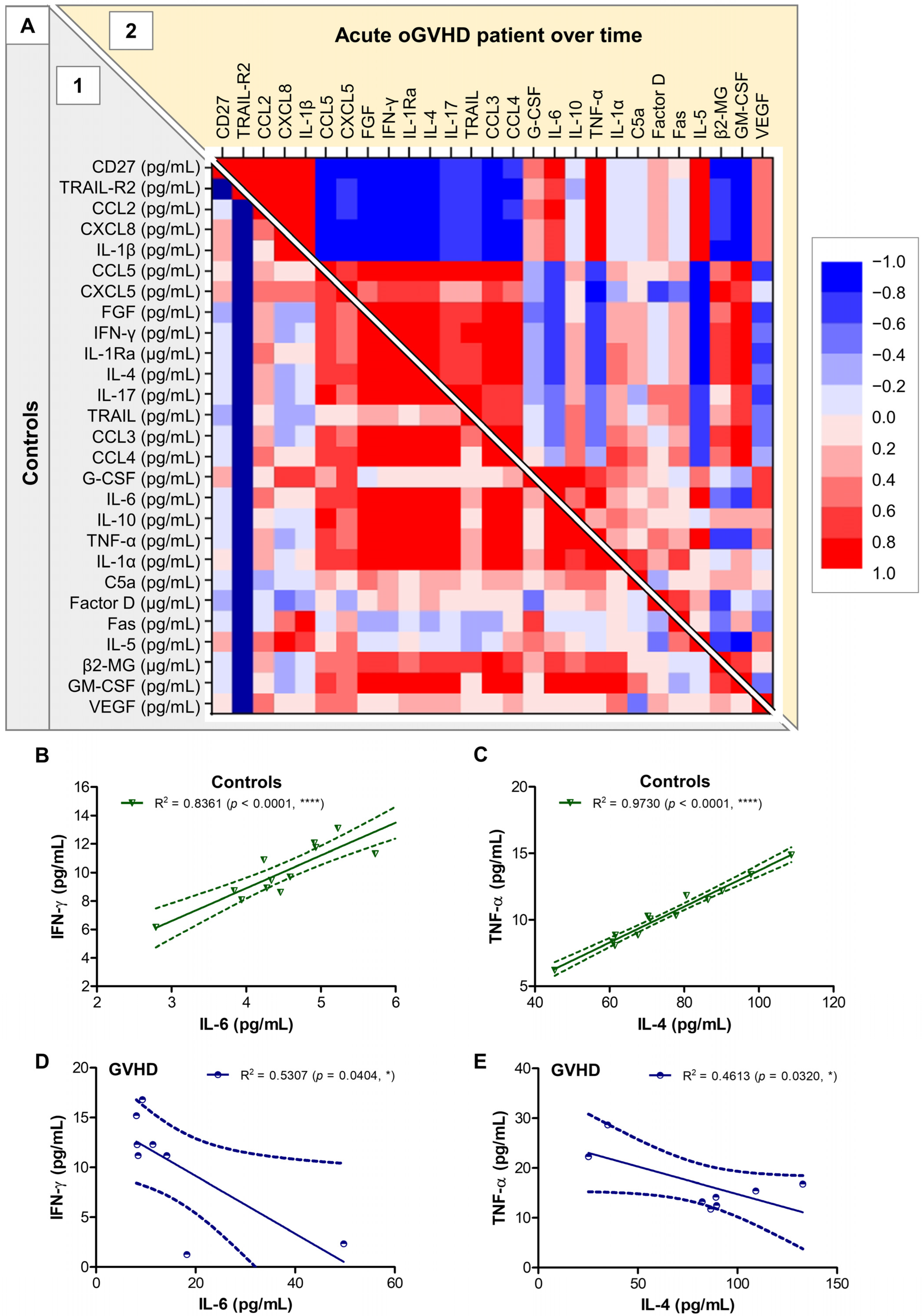
2.2. Tear Analysis
3. Discussion
3.1. Ophthalmological Examination Status
3.2. Tear Biomarkers
3.2.1. The First Group of Tear Biomarkers
3.2.2. The Second Group of Tear Biomarkers
3.2.3. The Third Group of Tear Biomarkers
3.3. Impact of Therapeutic Management on Clinical Outcome and Tear Biomarker Dynamics
3.3.1. Impact on Clinical Outcome
3.3.2. Impact on Tear Biomarkers
4. Materials and Methods
4.1. Human Study
4.2. Tear Sample Processing
4.3. Tear Cytokine Analysis
4.4. Statistical Analysis
5. Conclusions
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| oGVHD | Ocular graft versus host disease |
| cGVHD | Chronic graft versus host disease |
| RE | Right eye |
| LE | Left eye |
| β2-MG | beta2-microglobulin |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TRAIL-R2 | TRAIL receptor 2 |
| CCL2/MCP-1 | Chemokine ligand 2/Monocyte chemoattractant protein-1 |
| CCL3/MIP-1α | Chemokine ligand 3/Macrophage inflammatory protein 1-alpha |
| CCL4/MIP-1β | Chemokine ligand 4/Macrophage inflammatory protein 1-beta |
| CCL5/RANTES | Chemokine ligand 5/Regulated upon Activation, Normal T cell Expressed and presumably Secreted |
| CXCL5/ENA-78 | C-X-C motif chemokine 5/Epithelial cell-derived neutrophil-activating peptide |
| CXCL8/IL-8 | C-X-C motif chemokine 5/Interleukin 8 |
| FGF | Fibroblast growth factor |
| VEGF | Vascular endothelial growth factor |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| TNF-α | Tumor necrosis factor-alpha |
| IFN-γ | Interferon gamma |
| IL-1Ra | Interleukin-1 receptor antagonist |
| sem | Standard error of the mean |
References
- Gratwohl, A.; Pasquini, M.C.; Aljurf, M.; Atsuta, Y.; Baldomero, H.; Foeken, L.; Gratwohl, M.; Bouzas, L.F.; Confer, D.; Frauendorfer, K.; et al. One Million Haemopoietic Stem-Cell Transplants: A Retrospective Observational Study. Lancet Haematol. 2015, 2, e91–e100. [Google Scholar] [CrossRef] [PubMed]
- Justiz Vaillant, A.A.; Modi, P.; Mohammadi, O. Graft-Versus-Host Disease; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Carreno-Galeano, J.T.; Dohlman, T.H.; Kim, S.; Yin, J.; Dana, R. A Review of Ocular Graft-versus-Host Disease: Pathophysiology, Clinical Presentation and Management. Ocul. Immunol. Inflamm. 2021, 29, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Cho, W.; Liu, C.; Naderi, A.; Surico, P.L.; Kahale, F.; Dohlman, T.H.; Chauhan, S.K.; Dana, R. Immunopathological Mechanisms and Clinical Manifestations of Ocular Graft-versus-Host Disease Following Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant. 2024, 59, 1049–1056. [Google Scholar] [CrossRef]
- Ban, Y.; Ogawa, Y.; Ibrahim, O.M.A.; Tatematsu, Y.; Kamoi, M.; Uchino, M.; Yaguchi, S.; Dogru, M.; Tsubota, K. Morphologic Evaluation of Meibomian Glands in Chronic Graft-versus-Host Disease Using in Vivo Laser Confocal Microscopy. Mol. Vis. 2011, 17, 2533–2543. [Google Scholar]
- Inamoto, Y.; Valdés-Sanz, N.; Ogawa, Y.; Alves, M.; Berchicci, L.; Galvin, J.; Greinix, H.; Hale, G.A.; Horn, B.; Kelly, D.; et al. Ocular Graft-versus-Host Disease after Hematopoietic Cell Transplantation: Expert Review from the Late Effects and Quality of Life Working Committee of the CIBMTR and Transplant Complications Working Party of the EBMT. Bone Marrow Transplant. 2019, 54, 662–673. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, R.; Huang, S.; Fan, W.; Yuan, R.; Wang, X.; Zhang, X. Recent Advances in Ocular Graft-versus-Host Disease. Front. Immunol. 2023, 14, 1092108. [Google Scholar] [CrossRef]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Bernabei, F.; Barbato, F.; Arpinati, M.; Giannaccare, G.; Versura, P.; Bonifazi, F. Incidence, Risk Factors and Complications of Ocular Graft-Versus-Host Disease Following Hematopoietic Stem Cell Transplantation. Am. J. Ophthalmol. 2021, 227, 25–34. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Tamhane, M.; Cabrera-Ghayouri, S.; Abelian, G.; Viswanath, V. Review of Biomarkers in Ocular Matrices: Challenges and Opportunities. Pharm. Res. 2019, 36, 40. [Google Scholar] [CrossRef]
- Pavel-Tanasa, M.; Constantinescu, D.; Cianga, C.M.; Anisie, E.; Mereuta, A.I.; Tuchilus, C.G.; Cianga, P. Adipokines, and Not Vitamin D, Associate with Antibody Immune Responses Following Dual BNT162b2 Vaccination within Individuals Younger than 60 Years. Front. Immunol. 2022, 13, 1000006. [Google Scholar] [CrossRef]
- Saleki, K.; Shirzad, M.; Javanian, M.; Mohammadkhani, S.; Alijani, M.H.; Miri, N.; Oladnabi, M.; Azadmehr, A. Serum Soluble Fas Ligand Is a Severity and Mortality Prognostic Marker for COVID-19 Patients. Front. Immunol. 2022, 13, 947401. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Arakaki, R.; Saito, M.; Kudo, Y.; Ishimaru, N. Dual Role of Fas/FasL-Mediated Signal in Peripheral Immune Tolerance. Front. Immunol. 2017, 8, 403. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Murakami, Y.; Kataoka, K.; Notomi, S.; Mantopoulos, D.; Trichonas, G.; Miller, J.W.; Gregory, M.S.; Ksander, B.R.; Marshak-Rothstein, A.; et al. Membrane-Bound and Soluble Fas Ligands Have Opposite Functions in Photoreceptor Cell Death Following Separation from the Retinal Pigment Epithelium. Cell Death Dis. 2015, 6, e1986. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.; Singh, P.; Tengryd, C.; Cavalera, M.; Yao Mattisson, I.; Nitulescu, M.; Flor Persson, A.; Volkov, P.; Engström, G.; Orho-Melander, M.; et al. STRAIL-R2 (Soluble TNF [Tumor Necrosis Factor]-Related Apoptosis-Inducing Ligand Receptor 2) a Marker of Plaque Cell Apoptosis and Cardiovascular Events. Stroke 2019, 50, 1989–1996. [Google Scholar] [CrossRef]
- Staniek, J.; Lorenzetti, R.; Heller, B.; Janowska, I.; Schneider, P.; Unger, S.; Warnatz, K.; Seidl, M.; Venhoff, N.; Thiel, J.; et al. TRAIL-R1 and TRAIL-R2 Mediate TRAIL-Dependent Apoptosis in Activated Primary Human B Lymphocytes. Front. Immunol. 2019, 10, 951. [Google Scholar] [CrossRef]
- Naval, J.; de Miguel, D.; Gallego-Lleyda, A.; Anel, A.; Martinez-Lostao, L. Importance of TRAIL Molecular Anatomy in Receptor Oligomerization and Signaling. Implications for Cancer Therapy. Cancers 2019, 11, 444. [Google Scholar] [CrossRef]
- Fenton, M.J.; Buras, J.A.; Donnelly, R.P. IL-4 Reciprocally Regulates IL-1 and IL-1 Receptor Antagonist Expression in Human Monocytes. J. Immunol. 1992, 149, 1283–1288. [Google Scholar] [CrossRef]
- Mühl, H.; Pfeilschifter, J. Anti-Inflammatory Properties of pro-Inflammatory Interferon-Gamma. Int. Immunopharmacol. 2003, 3, 1247–1255. [Google Scholar] [CrossRef]
- Falschlehner, C.; Schaefer, U.; Walczak, H. Following TRAIL’s Path in the Immune System. Immunology 2009, 127, 145–154. [Google Scholar] [CrossRef]
- Janjic, B.M.; Kulkarni, A.; Ferris, R.L.; Vujanovic, L.; Vujanovic, N.L. Human B Cells Mediate Innate Anti-Cancer Cytotoxicity Through Concurrent Engagement of Multiple TNF Superfamily Ligands. Front. Immunol. 2022, 13, 837842. [Google Scholar] [CrossRef]
- Grisanti, L.A. TRAIL and Its Receptors in Cardiac Diseases. Front. Physiol. 2023, 14, 1256852. [Google Scholar] [CrossRef]
- Nguyen, H.; Alawieh, A.; Bastian, D.; Kuril, S.; Dai, M.; Daenthanasanmak, A.; Zhang, M.; Iamsawat, S.; Schutt, S.D.; Wu, Y.; et al. Targeting the Complement Alternative Pathway Permits Graft Versus Leukemia Activity While Preventing Graft Versus Host Disease. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 3481–3490. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, D.A.; Gavriilaki, E.; Chanou, I.; Meyer, S.C. Hemostasis and Complement in Allogeneic Hematopoietic Stem Cell Transplantation: Clinical Significance of Two Interactive Systems. Bone Marrow Transplant. 2024, 59, 1349–1359. [Google Scholar] [CrossRef]
- Speidl, W.S.; Kastl, S.P.; Hutter, R.; Katsaros, K.M.; Kaun, C.; Bauriedel, G.; Maurer, G.; Huber, K.; Badimon, J.J.; Wojta, J. The Complement Component C5a Is Present in Human Coronary Lesions in Vivo and Induces the Expression of MMP-1 and MMP-9 in Human Macrophages in Vitro. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 35–44. [Google Scholar] [CrossRef]
- Bystry, R.S.; Aluvihare, V.; Welch, K.A.; Kallikourdis, M.; Betz, A.G. B Cells and Professional APCs Recruit Regulatory T Cells via CCL4. Nat. Immunol. 2001, 2, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Reshef, R.; Luger, S.M.; Hexner, E.O.; Loren, A.W.; Frey, N.V.; Nasta, S.D.; Goldstein, S.C.; Stadtmauer, E.A.; Smith, J.; Bailey, S.; et al. Blockade of Lymphocyte Chemotaxis in Visceral Graft-versus-Host Disease. N. Engl. J. Med. 2012, 367, 135–145. [Google Scholar] [CrossRef]
- Morikawa, K.; Miyawaki, T.; Oseko, F.; Morikawa, S.; Imai, K. G-CSF Enhances the Immunoglobulin Generation Rather than the Proliferation of Human B Lymphocytes. Eur. J. Haematol. 1993, 51, 144–151. [Google Scholar] [CrossRef]
- Itoh, K.; Hirohata, S. The Role of IL-10 in Human B Cell Activation, Proliferation, and Differentiation. J. Immunol. 1995, 154, 4341–4350. [Google Scholar] [CrossRef]
- Lighaam, L.C.; Unger, P.-P.A.; Vredevoogd, D.W.; Verhoeven, D.; Vermeulen, E.; Turksma, A.W.; Ten Brinke, A.; Rispens, T.; van Ham, S.M. In Vitro-Induced Human IL-10(+) B Cells Do Not Show a Subset-Defining Marker Signature and Plastically Co-Express IL-10 With Pro-Inflammatory Cytokines. Front. Immunol. 2018, 9, 1913. [Google Scholar] [CrossRef]
- Nova-Lamperti, E.; Fanelli, G.; Becker, P.D.; Chana, P.; Elgueta, R.; Dodd, P.C.; Lord, G.M.; Lombardi, G.; Hernandez-Fuentes, M.P. IL-10-Produced by Human Transitional B-Cells down-Regulates CD86 Expression on B-Cells Leading to Inhibition of CD4+T-Cell Responses. Sci. Rep. 2016, 6, 20044. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.H.; Ley, T.J. Lymphocyte-Mediated Cytotoxicity. Annu. Rev. Immunol. 2002, 20, 323–370. [Google Scholar] [CrossRef]
- Du, W.; Cao, X. Cytotoxic Pathways in Allogeneic Hematopoietic Cell Transplantation. Front. Immunol. 2018, 9, 2979. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Hatsumi, N.; Saito, T.; Matsushima, T.; Sakura, T.; Tamura, J.; Karasawa, M.; Miyawaki, S. Two Cases of Chronic Graft-versus-Host Disease with Elevated Levels of Soluble Fas Ligand in Serum. Am. J. Hematol. 2000, 64, 133–136. [Google Scholar] [CrossRef]
- Kim, S.K. Ocular Graft vs. Host Disease. Ocul. Surf. 2005, 3, S177–S179. [Google Scholar] [CrossRef]
- Masalkhi, M.; Wahoud, N.; Moran, B.; Elhassadi, E. Evolving Therapeutic Paradigms in Ocular Graft-versus-Host Disease. Eye 2024, 38, 3215–3217. [Google Scholar] [CrossRef]
- Royer, D.J.; Echegaray-Mendez, J.; Lin, L.; Gmyrek, G.B.; Mathew, R.; Saban, D.R.; Perez, V.L.; Carr, D.J. Complement and CD4(+) T Cells Drive Context-Specific Corneal Sensory Neuropathy. Elife 2019, 8, e48378. [Google Scholar] [CrossRef]
- Tappeiner, C.; Heiligenhaus, A.; Halter, J.P.; Miserocchi, E.; Bandello, F.; Goldblum, D. Challenges and Concepts in the Diagnosis and Management of Ocular Graft-versus-Host Disease. Front. Med. 2023, 10, 1133381. [Google Scholar] [CrossRef] [PubMed]
- Paranga, T.G.; Pavel-Tanasa, M.; Constantinescu, D.; Iftimi, E.; Plesca, C.E.; Miftode, I.-L.; Cianga, P.; Miftode, E. Distinct Soluble Immune Checkpoint Profiles Characterize COVID-19 Severity, Mortality and SARS-CoV-2 Variant Infections. Front. Immunol. 2024, 15, 1464480. [Google Scholar] [CrossRef]
- Roberts, A.I.; Devadas, S.; Zhang, X.; Zhang, L.; Keegan, A.; Greeneltch, K.; Solomon, J.; Wei, L.; Das, J.; Sun, E.; et al. The Role of Activation-Induced Cell Death in the Differentiation of T-Helper-Cell Subsets. Immunol. Res. 2003, 28, 285–293. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhang, L.Y.; Devadas, S.; Li, L.; Keegan, A.D.; Shi, Y.F. Reciprocal Expression of TRAIL and CD95L in Th1 and Th2 Cells: Role of Apoptosis in T Helper Subset Differentiation. Cell Death Differ. 2003, 10, 203–210. [Google Scholar] [CrossRef]
- Shen, Z.; Ma, J.; Peng, R.; Hu, B.; Zhao, Y.; Liu, S.; Hong, J. Biomarkers in Ocular Graft-Versus-Host Disease: Implications for the Involvement of B Cells. Transplant. Cell. Ther. 2022, 28, 749.e1–749.e7. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, H.; Fang, J.; Xie, W.; Hong, M.; Xia, L. Elevated Fas/FasL System and Endothelial Cell Microparticles Are Involved in Endothelial Damage in Acute Graft-versus-Host Disease: A Clinical Analysis. Leuk. Res. 2012, 36, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Martinez-Guasch, F.; Sajjan, S.; Cao, X.; Atanackovic, D.; Sunshine, S. Tear Cytokine Profiling for Ocular Graft versus Host Disease Utilizing a New Multiplex Assay. J. Immunol. 2023, 210, 173.41. [Google Scholar] [CrossRef]
- Antin, J.H.; Weisdorf, D.; Neuberg, D.; Nicklow, R.; Clouthier, S.; Lee, S.J.; Alyea, E.; McGarigle, C.; Blazar, B.R.; Sonis, S.; et al. Interleukin-1 Blockade Does Not Prevent Acute Graft-versus-Host Disease: Results of a Randomized, Double-Blind, Placebo-Controlled Trial of Interleukin-1 Receptor Antagonist in Allogeneic Bone Marrow Transplantation. Blood 2002, 100, 3479–3482. [Google Scholar] [CrossRef]
- Cocho, L.; Fernández, I.; Calonge, M.; Martínez, V.; González-García, M.J.; Caballero, D.; López-Corral, L.; García-Vázquez, C.; Vázquez, L.; Stern, M.E.; et al. Biomarkers in Ocular Chronic Graft Versus Host Disease: Tear Cytokine- and Chemokine-Based Predictive Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 746–758. [Google Scholar] [CrossRef]
- Fan, N.-W.; Dohlman, T.H.; Foulsham, W.; McSoley, M.; Singh, R.B.; Chen, Y.; Dana, R. The Role of Th17 Immunity in Chronic Ocular Surface Disorders. Ocul. Surf. 2021, 19, 157–168. [Google Scholar] [CrossRef]
- Disteldorf, E.M.; Krebs, C.F.; Paust, H.-J.; Turner, J.-E.; Nouailles, G.; Tittel, A.; Meyer-Schwesinger, C.; Stege, G.; Brix, S.; Velden, J.; et al. CXCL5 Drives Neutrophil Recruitment in TH17-Mediated GN. J. Am. Soc. Nephrol. 2015, 26, 55–66. [Google Scholar] [CrossRef]
- Choi, W.; Li, Z.; Oh, H.-J.; Im, S.-K.; Lee, S.-H.; Park, S.-H.; You, I.-C.; Yoon, K.-C. Expression of CCR5 and Its Ligands CCL3, -4, and -5 in the Tear Film and Ocular Surface of Patients with Dry Eye Disease. Curr. Eye Res. 2012, 37, 12–17. [Google Scholar] [CrossRef]
- Pietraszkiewicz, A.A.; Payne, D.; Abraham, M.; Garced, A.; Devarasetty, K.C.; Wall, M.; Menezes, S.M.; Ugarte, S.; Pirsl, F.; Goklemez, S.; et al. Ocular Surface Indicators and Biomarkers in Chronic Ocular Graft-versus-Host Disease: A Prospective Cohort Study. Bone Marrow Transplant. 2021, 56, 1850–1858. [Google Scholar] [CrossRef]
- Via, C.S.; Soloviova, K.; Puliaiev, M.; Puliav, R.; Puliaeva, I.; Morris, S.C.; Finkelman, F.D. In Vivo IL-4 Prevents Allo-Antigen Driven CD8+ CTL Development. Clin. Immunol. 2017, 180, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hill, G.R. Interleukin-10 Mediated Immune Regulation after Stem Cell Transplantation: Mechanisms and Implications for Therapeutic Intervention. Semin. Immunol. 2019, 44, 101322. [Google Scholar] [CrossRef]
- Panoskaltsis-Mortari, A.; Lacey, D.L.; Vallera, D.A.; Blazar, B.R. Keratinocyte Growth Factor Administered Before Conditioning Ameliorates Graft-Versus-Host Disease After Allogeneic Bone Marrow Transplantation in Mice. Blood 1998, 92, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, J.; Gomez, C.; Zhou, J.; Martinez Guasch, F.; Wandvik, C.; Sunshine, S.B. Molecular Biomarkers in Ocular Graft-versus-Host Disease: A Systematic Review. Biomolecules 2024, 14, 102. [Google Scholar] [CrossRef]
- Qiu, Y.; Hu, B.; Peng, R.-M.; Huang, J.-F.; Hong, J. Tear Cytokines as Biomarkers for Acute Ocular Graft-Versus-Host Disease. Cornea 2022, 41, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Borges, D.; Alborghetti, M.R.; Franco Paes Leme, A.; Ramos Domingues, R.; Duarte, B.; Veiga, M.; Trindade Ferrer, M.; Viana Wanzeler, A.C.; Leite Arieta, C.E.; Alves, M. Tear Proteomic Profile in Three Distinct Ocular Surface Diseases: Keratoconus, Pterygium, and Dry Eye Related to Graft-versus-Host Disease. Clin. Proteom. 2020, 17, 42. [Google Scholar] [CrossRef]
- Riesner, K.; Shi, Y.; Jacobi, A.; Kräter, M.; Kalupa, M.; McGearey, A.; Mertlitz, S.; Cordes, S.; Schrezenmeier, J.-F.; Mengwasser, J.; et al. Initiation of Acute Graft-versus-Host Disease by Angiogenesis. Blood 2017, 129, 2021–2032. [Google Scholar] [CrossRef]
- Penack, O.; Socié, G.; van den Brink, M.R.M. The Importance of Neovascularization and Its Inhibition for Allogeneic Hematopoietic Stem Cell Transplantation. Blood 2011, 117, 4181–4189. [Google Scholar] [CrossRef]
- Shikari, H.; Antin, J.H.; Dana, R. Ocular Graft-versus-Host Disease: A Review. Surv. Ophthalmol. 2013, 58, 233–251. [Google Scholar] [CrossRef]
- Zhang, M.-Y.; Zhao, P.; Zhang, Y.; Wang, J.-S. Efficacy and Safety of Ruxolitinib for Steroid-Refractory Graft-versus-Host Disease: Systematic Review and Meta-Analysis of Randomised and Non-Randomised Studies. PLoS ONE 2022, 17, e0271979. [Google Scholar] [CrossRef]
- Hill, G.R.; Koyama, M. Cytokines and Costimulation in Acute Graft-versus-Host Disease. Blood 2020, 136, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Henden, A.S.; Hill, G.R. Cytokines in Graft-versus-Host Disease. J. Immunol. 2015, 194, 4604–4612. [Google Scholar] [CrossRef]
- Yu, Y.; Zhong, J.; Peng, L.; Wang, B.; Li, S.; Huang, H.; Deng, Y.; Zhang, H.; Yang, R.; Wang, C.; et al. Tacrolimus Downregulates Inflammation by Regulating Pro_/Anti_inflammatory Responses in LPS_induced Keratitis. Mol. Med. Rep. 2017, 16, 5855–5862. [Google Scholar] [CrossRef] [PubMed]
- Nash, R.A.; Antin, J.H.; Karanes, C.; Fay, J.W.; Avalos, B.R.; Yeager, A.M.; Przepiorka, D.; Davies, S.; Petersen, F.B.; Bartels, P.; et al. Phase 3 Study Comparing Methotrexate and Tacrolimus with Methotrexate and Cyclosporine for Prophylaxis of Acute Graft-versus-Host Disease after Marrow Transplantation from Unrelated Donors. Blood 2000, 96, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Surico, P.L.; Luo, Z.K. Understanding Ocular Graft-versus-Host Disease to Facilitate an Integrated Multidisciplinary Approach. Transplant. Cell. Ther. 2024, 30, S570–S584. [Google Scholar] [CrossRef]
- Hessen, M.; Akpek, E.K. Ocular Graft-versus-Host Disease. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 540–547. [Google Scholar] [CrossRef]
- Quintás-Cardama, A.; Vaddi, K.; Liu, P.; Manshouri, T.; Li, J.; Scherle, P.A.; Caulder, E.; Wen, X.; Li, Y.; Waeltz, P.; et al. Preclinical Characterization of the Selective JAK1/2 Inhibitor INCB018424: Therapeutic Implications for the Treatment of Myeloproliferative Neoplasms. Blood 2010, 115, 3109–3117. [Google Scholar] [CrossRef]
- Zeiser, R.; von Bubnoff, N.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef]
- Choi, J.; Cooper, M.L.; Alahmari, B.; Ritchey, J.; Collins, L.; Holt, M.; DiPersio, J.F. Pharmacologic Blockade of JAK1/JAK2 Reduces GvHD and Preserves the Graft-versus-Leukemia Effect. PLoS ONE 2014, 9, e109799. [Google Scholar] [CrossRef]
- Hechinger, A.-K.; Smith, B.A.H.; Flynn, R.; Hanke, K.; McDonald-Hyman, C.; Taylor, P.A.; Pfeifer, D.; Hackanson, B.; Leonhardt, F.; Prinz, G.; et al. Therapeutic Activity of Multiple Common γ-Chain Cytokine Inhibition in Acute and Chronic GVHD. Blood 2015, 125, 570–580. [Google Scholar] [CrossRef]
- Yeleswaram, S.; Smith, P.; Burn, T.; Covington, M.; Juvekar, A.; Li, Y.; Squier, P.; Langmuir, P. Inhibition of Cytokine Signaling by Ruxolitinib and Implications for COVID-19 Treatment. Clin. Immunol. 2020, 218, 108517. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, S.; Bhatt, V.; Galvin, J.; Tian, C.; Zieser, R. Oral Ruxolitinib Treatment for Patients with Ocular Manifestations of Chronic Graft-versus-Host Disease: A Post Hoc Analysis of the Phase 3 REACH3 Study. Investig. Ophthalmol. Vis. Sci. 2023, 64, 1937. [Google Scholar]
- Sajjan, S.; Tibbs, E.; Utz, M.; Rapoport, A.P.; Yared, J.; Dahiya, S.; Cao, X.; Hardy, N.; Sunshine, S.B. Can Janus Kinase Inhibition Improve Ocular Graft versus Host Disease? Ocul. Surf. 2023, 28, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.; Tabbara, K.F.; Aljurf, M. Ocular Manifestations of Graft-versus-Host Disease. Saudi J. Ophthalmol. Off. J. Saudi Ophthalmol. Soc. 2013, 27, 215–222. [Google Scholar] [CrossRef]





| Variable | aGVHD RE T0 | aGVHD LE T0 | aGVHD RE T1 | aGVHD LE T1 | aGVHD RE T2 | aGVHD LE T2 | cGVHD RE T0 | cGVHD LE T0 | Controls Mean ± S.E.M. |
|---|---|---|---|---|---|---|---|---|---|
| Schirmer I test (mm) | 30 | 25 | 8 | 7 | 5 | 5 | 12 | 7 | 24.15 ± 2.68 |
| Proteins (mg/mL) | 2.59 | 2.95 | 1.15 | 0.93 | 0.77 | 0.92 | 1.03 | 1.01 | 1.17 ± 0.09 |
| β2-MG (μg/mL) | 103.89 | 58.71 | 227.88 | 230.27 | 190.05 | 122.98 | 307.86 | 284.64 | 258.52 ± 15.7 |
| C5a (pg/mL) | 46.52 | 30.81 | 32.07 | 45.59 | 65.09 | 24.1 | 5.9 | 20.14 | 16.86 ± 1.17 |
| CD27 (pg/mL) | 297.79 | 288.13 | 0 | 0.79 | 0 | 20.29 | 71.05 | 125.89 | 0.93 ± 0.93 |
| Factor D (μg/mL) | 13.36 | 12.01 | 4.5 | 4.64 | 10.01 | 17.53 | 9 | 13.17 | 3.1 ± 0.36 |
| Fas (pg/mL) | 956.98 | 963.82 | 640.23 | 562.92 | 1337.4 | 1316.85 | 703.62 | 1080.14 | 2.69 ± 2.69 |
| TRAIL-R2 (pg/mL) | 95.12 | 99.99 | 13.29 | 16.52 | 0 | 0 | 0 | 0 | 0 ± 0 |
| TRAIL (pg/mL) | 79.83 | 25.26 | 166.37 | 73.81 | 146.33 | 216.89 | 642.33 | 470.32 | 1003.19 ± 186.47 |
| CCL2 (pg/mL) | 658.07 | 564 | 48.4 | 70.99 | 9.33 | 10.86 | 58.45 | 30.08 | 14.17 ± 2.99 |
| CCL3 (pg/mL) | 291.65 | 115.29 | 447.32 | 497.2 | 646.18 | 563.95 | 559.68 | 541.16 | 423.23 ± 25.94 |
| CCL4 (pg/mL) | 42.22 | 20.61 | 47.94 | 50.42 | 70.45 | 59.97 | 51.2 | 55 | 45.35 ± 2.44 |
| CCL5 (pg/mL) | 15.03 | 9.56 | 31.62 | 35.13 | 44.66 | 47.51 | 88.3 | 84.41 | 31.49 ± 2.51 |
| CXCL5 (pg/mL) | 58.08 | 46.56 | 174.39 | 224.25 | 127.25 | 123.98 | 299.65 | 189.8 | 82.48 ± 5 |
| CXCL8 (pg/mL) | 728.86 | 760.84 | 195.89 | 193.93 | 143.5 | 154.51 | 457.47 | 539.93 | 60.3 ± 17.7 |
| FGF (pg/mL) | 76.84 | 103.61 | 228.11 | 234.36 | 329.55 | 301.72 | 227.17 | 240.08 | 199.62 ± 12.58 |
| G-CSF (pg/mL) | 103.4 | 1.36 | 23.1 | 27.08 | 28.57 | 22.14 | 136.44 | 148.74 | 16.13 ± 2.63 |
| GM-CSF (pg/mL) | 7.34 | 4.94 | 24.78 | 24.87 | 37.01 | 31.58 | 26.47 | 23.51 | 19.49 ± 1.31 |
| IFN-γ (pg/mL) | 2.35 | 1.26 | 12.28 | 11.19 | 16.81 | 15.19 | 11.2 | 12.3 | 10.29 ± 0.66 |
| IL-1α (pg/mL) | 70.49 | 58.41 | 56.54 | 61.21 | 79.75 | 65.49 | 57.46 | 60.06 | 49.77 ± 2.88 |
| IL-1β (pg/mL) | 736.01 | 777.18 | 16.4 | 35.12 | 15.29 | 14.01 | 65.9 | 116.62 | 7.74 ± 1.89 |
| IL-1Ra (pg/mL) | 9.27 | 6.6 | 67.13 | 72.67 | 119.92 | 92.34 | 57.77 | 63.48 | 55.95 ± 4.59 |
| IL-4 (pg/mL) | 34.88 | 25.22 | 86.58 | 89.58 | 132.83 | 109.2 | 82.27 | 89.24 | 75.35 ± 4.8 |
| IL-5 (pg/mL) | 0.26 | 0.25 | 0.17 | 0.16 | 0.19 | 0.16 | 0.004 | 0.21 | 0.07 ± 0.11 |
| IL-6 (pg/mL) | 49.76 | 18.26 | 8.24 | 8.46 | 9.31 | 8.11 | 14.2 | 11.41 | 4.61 ± 0.26 |
| IL-10 (pg/mL) | 108.96 | 12.33 | 61.17 | 63.69 | 76.52 | 76.12 | 72.38 | 73.59 | 43.97 ± 2.92 |
| IL-17 (pg/mL) | 0.04 | 0.01 | 3.42 | 3.51 | 6.89 | 15.14 | 3.06 | 2.53 | 1.74 ± 0.23 |
| TNF-α(pg/mL) | 28.65 | 21.29 | 11.73 | 12.48 | 17.77 | 15.43 | 13.26 | 14.15 | 10.37 ± 0.66 |
| VEGF (pg/mL) | 53.55 | 28.81 | 40.8 | 32.96 | 24.19 | 15.76 | 65.11 | 49.07 | 112.81 ± 9.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofte-Zorila, M.-M.; Pavel-Tanasa, M.; Constantinescu, D.; Cianga, C.; Branisteanu, D.C.; Giannaccare, G.; Lixi, F.; Dascalescu, A.; Vlas, N.; Turcas, S.; et al. Novel Tear Biomarkers in Ocular Graft Versus Host Disease Associated with Th1/Th2 Immune Responses: A Case Series and Literature Review. Int. J. Mol. Sci. 2025, 26, 4311. https://doi.org/10.3390/ijms26094311
Timofte-Zorila M-M, Pavel-Tanasa M, Constantinescu D, Cianga C, Branisteanu DC, Giannaccare G, Lixi F, Dascalescu A, Vlas N, Turcas S, et al. Novel Tear Biomarkers in Ocular Graft Versus Host Disease Associated with Th1/Th2 Immune Responses: A Case Series and Literature Review. International Journal of Molecular Sciences. 2025; 26(9):4311. https://doi.org/10.3390/ijms26094311
Chicago/Turabian StyleTimofte-Zorila, Mihaela-Madalina, Mariana Pavel-Tanasa, Daniela Constantinescu, Corina Cianga, Daniel Constantin Branisteanu, Giuseppe Giannaccare, Filippo Lixi, Angela Dascalescu, Nicoleta Vlas, Sabina Turcas, and et al. 2025. "Novel Tear Biomarkers in Ocular Graft Versus Host Disease Associated with Th1/Th2 Immune Responses: A Case Series and Literature Review" International Journal of Molecular Sciences 26, no. 9: 4311. https://doi.org/10.3390/ijms26094311
APA StyleTimofte-Zorila, M.-M., Pavel-Tanasa, M., Constantinescu, D., Cianga, C., Branisteanu, D. C., Giannaccare, G., Lixi, F., Dascalescu, A., Vlas, N., Turcas, S., & Preda, C. (2025). Novel Tear Biomarkers in Ocular Graft Versus Host Disease Associated with Th1/Th2 Immune Responses: A Case Series and Literature Review. International Journal of Molecular Sciences, 26(9), 4311. https://doi.org/10.3390/ijms26094311









