Neuro-Ophthalmological Disorders Associated with Obstructive Sleep Apnoea
Abstract
1. Introduction
1.1. Diagnostic Protocol
1.2. Epidemiology
1.3. Impact on the Visual System
1.4. Mechanisms Linking OSA to Neuro-Ophthalmological Disorders
1.5. Aims of This Review
1.6. Data Collection
2. Pathophysiology of Obstructive Sleep Apnoea
2.1. Structural and Functional Mechanisms of Upper Airway Collapse
2.2. Sleep Fragmentation and Ventilatory Control in Obstructive Sleep Apnoea
2.3. Intermittent Hypoxia, Oxidative Stress, and Systemic Inflammation
3. Neuro-Ophthalmological Manifestations of Obstructive Sleep Apnoea
3.1. Optic Neuropathies and Papilledema
3.2. Glaucoma
3.3. Retinal Microvascular Changes
3.4. Visual Field Defects and Processing Abnormalities
3.5. Therapeutic Implications for Visual Function
3.6. Diagnostic Evaluation of Neuro-Ophthalmological Disorders in Obstructive Sleep Apnoea
4. Pathophysiological Mechanisms Linking Obstructive Sleep Apnoea and Neuro-Ophthalmological Disorders
4.1. Hypoxia-Induced Molecular Changes in Ocular Tissues
4.2. Intracranial Pressure Fluctuations
4.3. Endothelial Dysfunction and Vascular Dysregulation
4.4. Chronic Inflammation and Oxidative Stress
4.5. Neurodegenerative Pathways and Protective Mechanisms
5. Clinical Management Strategies
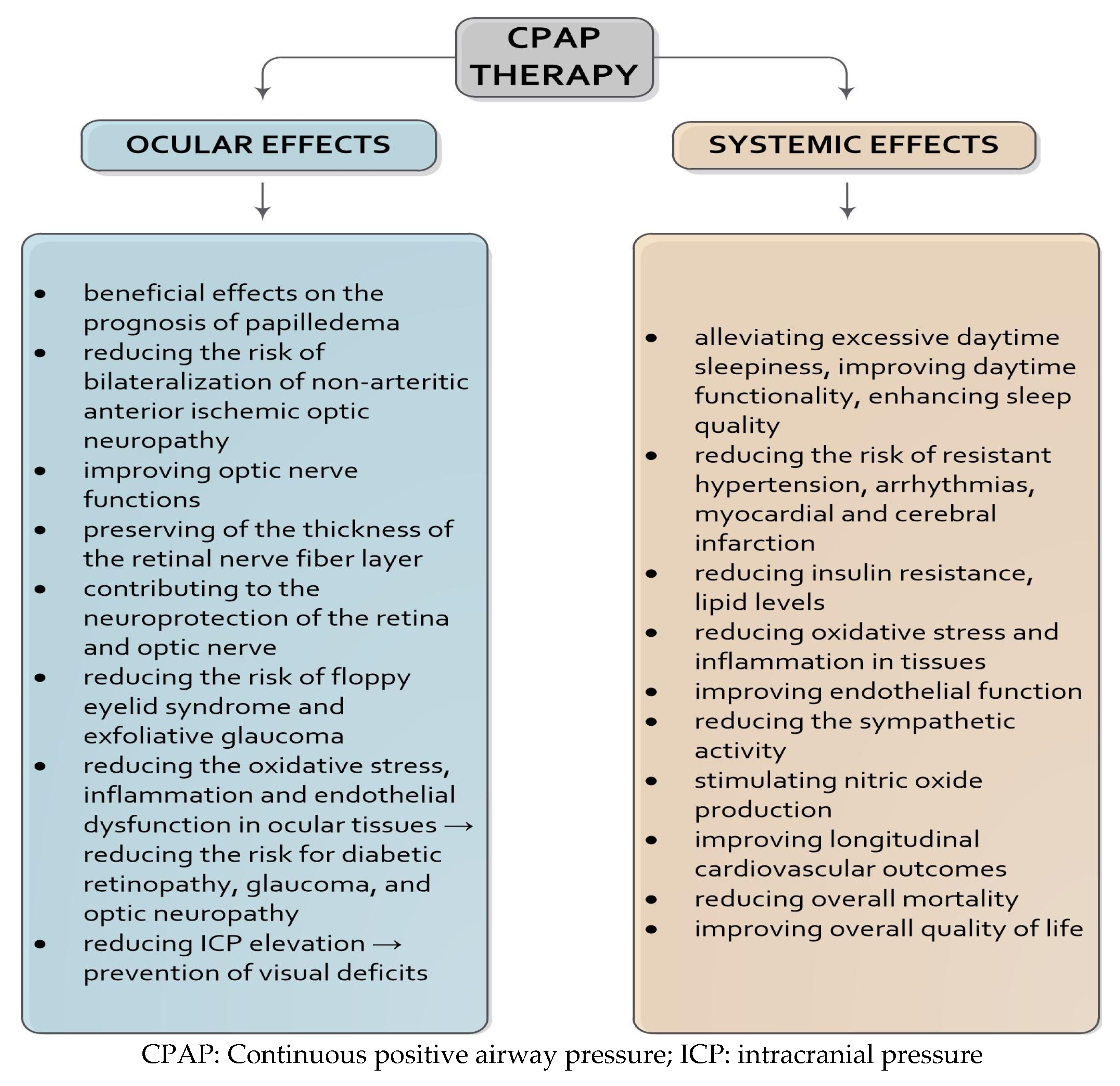
5.1. Continuous Positive Airway Pressure Therapy
5.2. Alternative Therapies: Surgical, Pharmacological, and Lifestyle Interventions
5.3. Antioxidants and Neuroprotective Agents
5.4. Diagnostic and Prognostic Significance of Polysomnography
5.5. Multidisciplinary Care and Patient-Centred Management
6. Prognostic Impact of CPAP Therapy on Neuro-Ophthalmological Outcomes
6.1. Neuroprotective Effects and Clinical Impact of CPAP Therapy
6.2. Adherence to CPAP and Disease Progression
6.3. Monitoring Strategies and the Role of OCT
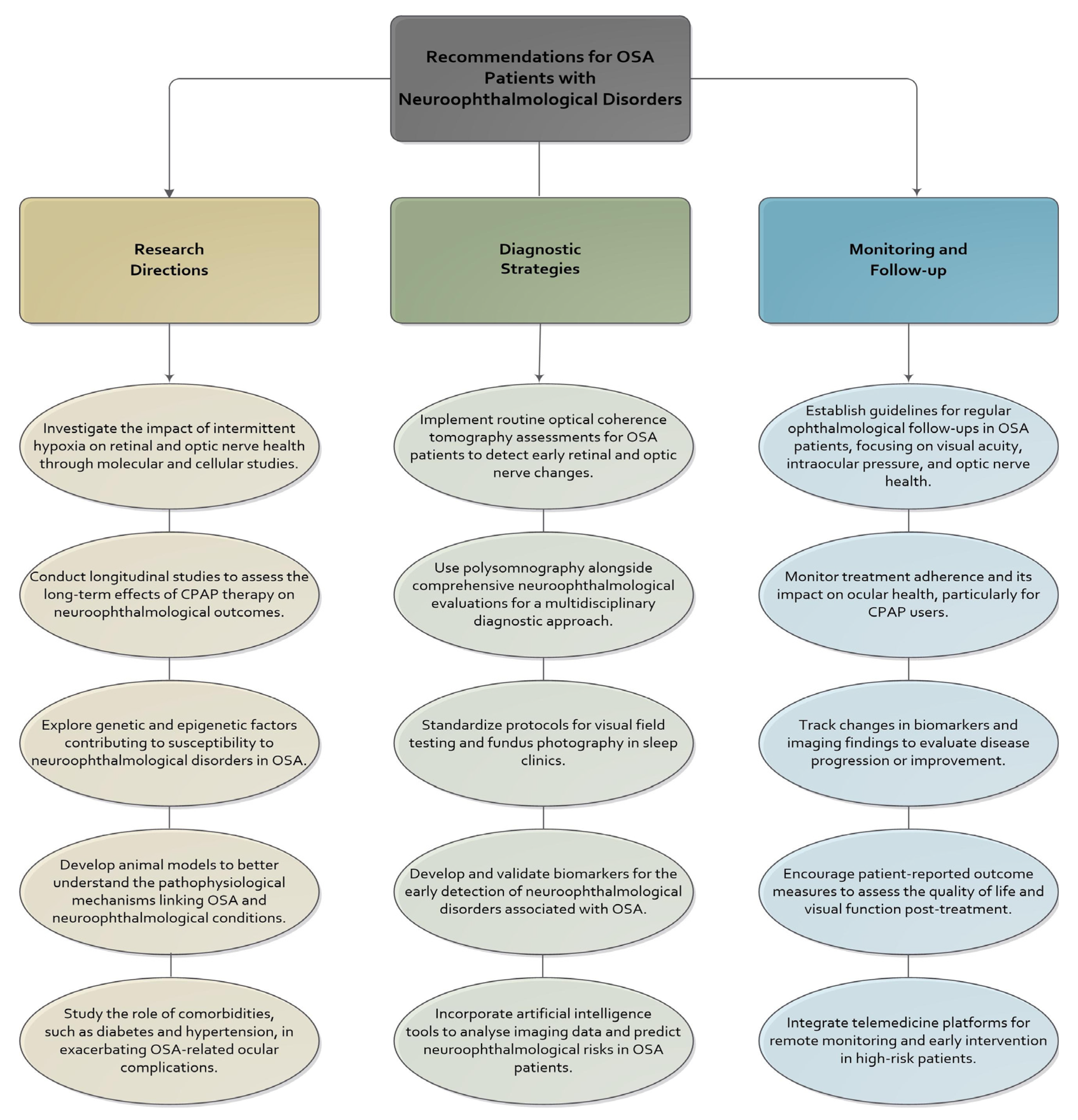
7. Future Directions and Research Opportunities
Recommendations for Clinical Practice and Future Research
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Berry, R.B.; Abreu, A.R.; Krishnan, V.; Quan, S.F.; Strollo, P.J.; Malhotra, R.K. A Transition to the American Academy of Sleep Medicine–Recommended Hypopnea Definition in Adults: Initiatives of the Hypopnea Scoring Rule Task Force. J. Clin. Sleep Med. 2022, 18, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.E.; Phillips, C.L.; Yee, B.J.; Grunstein, R.R. Obstructive Sleep Apnoea in Obesity: A Review. Clin. Obes. 2024, 14, e12651. [Google Scholar] [CrossRef] [PubMed]
- Pevernagie, D.A.; Gnidovec-Strazisar, B.; Grote, L.; Heinzer, R.; McNicholas, W.T.; Penzel, T.; Randerath, W.; Schiza, S.; Verbraecken, J.; Arnardottir, E.S. On the Rise and Fall of the Apnea−hypopnea Index: A Historical Review and Critical Appraisal. J. Sleep Res. 2020, 29, e13066. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Chen, P.-Y.; Chuang, L.-P.; Chen, N.-H.; Tu, Y.-K.; Hsieh, Y.-J.; Wang, Y.-C.; Guilleminault, C. Diagnostic Accuracy of the Berlin Questionnaire, STOP-BANG, STOP, and Epworth Sleepiness Scale in Detecting Obstructive Sleep Apnea: A Bivariate Meta-Analysis. Sleep Med. Rev. 2017, 36, 57–70. [Google Scholar] [CrossRef]
- Nikolopoulos, A.; Tatsis, K.; Tselepi, C.; Sioutkou, A.; Kostoulas, A.; Siopis, G.; Kostikas, K.; Konstantinidis, A. Quantifying the Sources of Discrepancy between Total Recording Time and Total Sleep Time in Home Sleep Apnea Testing: Insights from Home-Based Polysomnography. J. Clin. Sleep Med. 2025, 21, 1065–1072. [Google Scholar] [CrossRef]
- Bulloch, G.; Seth, I.; Zhu, Z.; Sukumar, S.; McNab, A. Ocular Manifestations of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 19–32. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of Obstructive Sleep Apnea in the General Population: A Systematic Review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Gulotta, G.; Iannella, G.; Vicini, C.; Polimeni, A.; Greco, A.; De Vincentiis, M.; Visconti, I.C.; Meccariello, G.; Cammaroto, G.; De Vito, A.; et al. Risk Factors for Obstructive Sleep Apnea Syndrome in Children: State of the Art. Int. J. Environ. Res. Public Health 2019, 16, 3235. [Google Scholar] [CrossRef]
- Rundo, J.V. Obstructive Sleep Apnea Basics. Cleve Clin. J. Med. 2019, 86, 2–9. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K.; et al. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-K.; Chiu, T.-Y.; Wang, N.-K.; Levi, S.R.; Tsai, M.-J. Ocular Complications of Obstructive Sleep Apnea. J. Clin. Med. 2021, 10, 3422. [Google Scholar] [CrossRef] [PubMed]
- Farahvash, A.; Micieli, J.A. Neuro-Ophthalmological Manifestations of Obstructive Sleep Apnea: Current Perspectives. Eye Brain 2020, 12, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Gorin, M.B. The Associations of Obstructive Sleep Apnea and Eye Disorders: Potential Insights into Pathogenesis and Treatment. Curr. Sleep Med. Rep. 2021, 7, 65–79. [Google Scholar] [CrossRef]
- Lv, R.; Liu, X.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological Mechanisms and Therapeutic Approaches in Obstructive Sleep Apnea Syndrome. Signal Transduct. Target. Ther. 2023, 8, 218. [Google Scholar] [CrossRef]
- Kurihara, T.; Westenskow, P.D.; Friedlander, M. Hypoxia-Inducible Factor (HIF)/Vascular Endothelial Growth Factor (VEGF) Signaling in the Retina. In Retinal Degenerative Diseases; Ash, J.D., Grimm, C., Hollyfield, J.G., Anderson, R.E., LaVail, M.M., Bowes Rickman, C., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; Volume 801, pp. 275–281. ISBN 978-1-4614-3208-1. [Google Scholar]
- Altaf, Q.A.; Dodson, P.; Ali, A.; Raymond, N.T.; Wharton, H.; Fellows, H.; Hampshire-Bancroft, R.; Shah, M.; Shepherd, E.; Miah, J.; et al. Obstructive Sleep Apnea and Retinopathy in Patients with Type 2 Diabetes. A Longitudinal Study. Am. J. Respir. Crit. Care Med. 2017, 196, 892–900. [Google Scholar] [CrossRef]
- Böhm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative Stress in the Eye and Its Role in the Pathophysiology of Ocular Diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef]
- Tang, B.; Li, S.; Cao, W.; Sun, X. The Association of Oxidative Stress Status with Open-Angle Glaucoma and Exfoliation Glaucoma: A Systematic Review and Meta-Analysis. J. Ophthalmol. 2019, 2019, 1803619. [Google Scholar] [CrossRef]
- Iorga, R.E.; Moraru, A.D.; Costin, D.; Munteanu-Dănulescu, R.S.; Brănișteanu, D.C. Current Trends in Targeting the Oxidative Stress in Glaucoma (Review). Eur. J. Ophthalmol. 2024, 34, 328–337. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef]
- Harańczyk, M.; Konieczyńska, M.; Płazak, W. Endothelial Dysfunction in Obstructive Sleep Apnea Patients. Sleep Breath. Schlaf Atm. 2022, 26, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Chen, Y.; Du, J. Obstructive Sleep Apnea Treatment in Adults. Kaohsiung J. Med. Sci. 2020, 36, 7–12. [Google Scholar] [CrossRef]
- Hyndych, A.; El-Abassi, R.; Mader, E.C. The Role of Sleep and the Effects of Sleep Loss on Cognitive, Affective, and Behavioral Processes. Cureus 2025, 17, e84232. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, W.; Jacobson, N.; Popplewell, N.; Moussavi, Z. Fluid-Structure Interaction Modelling of the Upper Airway with and without Obstructive Sleep Apnea: A Review. Med. Biol. Eng. Comput. 2022, 60, 1827–1849. [Google Scholar] [CrossRef] [PubMed]
- Hirata, R.P.; Schorr, F.; Kayamori, F.; Moriya, H.T.; Romano, S.; Insalaco, G.; Gebrim, E.M.; De Oliveira, L.V.F.; Genta, P.R.; Lorenzi-Filho, G. Upper Airway Collapsibility Assessed by Negative Expiratory Pressure While Awake Is Associated with Upper Airway Anatomy. J. Clin. Sleep Med. 2016, 12, 1339–1346. [Google Scholar] [CrossRef]
- Heinzer, R.C.; Stanchina, M.L.; Malhotra, A.; Fogel, R.B.; Patel, S.R.; Jordan, A.S.; Schory, K.; White, D.P. Lung Volume and Continuous Positive Airway Pressure Requirements in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 114–117. [Google Scholar] [CrossRef]
- Lee, J.J.; Sundar, K.M. Evaluation and Management of Adults with Obstructive Sleep Apnea Syndrome. Lung 2021, 199, 87–101. [Google Scholar] [CrossRef]
- Puri, S.; El-Chami, M.; Shaheen, D.; Ivers, B.; Panza, G.S.; Badr, M.S.; Lin, H.-S.; Mateika, J.H. Variations in Loop Gain and Arousal Threshold during NREM Sleep Are Affected by Time of Day over a 24-Hour Period in Participants with Obstructive Sleep Apnea. J. Appl. Physiol. 2020, 129, 800–809. [Google Scholar] [CrossRef]
- Oliven, R.; Cohen, G.; Somri, M.; Schwartz, A.R.; Oliven, A. Peri-Pharyngeal Muscle Response to Inspiratory Loading: Comparison of Patients with OSA and Healthy Subjects. J. Sleep Res. 2019, 28, e12756. [Google Scholar] [CrossRef]
- Sood, S.; Morrison, J.L.; Liu, H.; Horner, R.L. Role of Endogenous Serotonin in Modulating Genioglossus Muscle Activity in Awake and Sleeping Rats. Am. J. Respir. Crit. Care Med. 2005, 172, 1338–1347. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Yang, C.-C.; Tsai, C.-Y.; Majumdar, A.; Lee, K.-Y.; Feng, P.-H.; Tseng, C.-H.; Chen, K.-Y.; Kang, J.-H.; Lee, H.-C.; et al. Association of Low Arousal Threshold Obstructive Sleep Apnea Manifestations with Body Fat and Water Distribution. Life 2023, 13, 1218. [Google Scholar] [CrossRef] [PubMed]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent Hypoxemia and OSA. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HIF-1 and HIF-2 Transcription Factors—Similar but Not Identical. Mol. Cells 2010, 29, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gilkes, D.M. HIF-1 and HIF-2 in Cancer: Structure, Regulation, and Therapeutic Prospects. Cell. Mol. Life Sci. 2025, 82, 44. [Google Scholar] [CrossRef]
- Sánchez-de-la-Torre, M.; Gracia-Lavedan, E.; Benitez, I.D.; Sánchez-de-la-Torre, A.; Moncusí-Moix, A.; Torres, G.; Loffler, K.; Woodman, R.; Adams, R.; Labarca, G.; et al. Adherence to CPAP Treatment and the Risk of Recurrent Cardiovascular Events: A Meta-Analysis. JAMA 2023, 330, 1255. [Google Scholar] [CrossRef]
- Gagnon, K.; Baril, A.-A.; Gagnon, J.-F.; Fortin, M.; Décary, A.; Lafond, C.; Desautels, A.; Montplaisir, J.; Gosselin, N. Cognitive Impairment in Obstructive Sleep Apnea. Pathol. Biol. 2014, 62, 233–240. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, H.; Liu, H.; Xu, P. Role of Oxidative Stress in the Occurrence and Development of Cognitive Dysfunction in Patients with Obstructive Sleep Apnea Syndrome. Mol. Neurobiol. 2024, 61, 5083–5101. [Google Scholar] [CrossRef]
- Kohler, M.; Stradling, J.R. Mechanisms of Vascular Damage in Obstructive Sleep Apnea. Nat. Rev. Cardiol. 2010, 7, 677–685. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Xu, H.; Yi, H.; Guan, J.; Yin, S. Metabolomics and Microbiome Profiling as Biomarkers in Obstructive Sleep Apnoea: A Comprehensive Review. Eur. Respir. Rev. 2021, 30, 200220. [Google Scholar] [CrossRef]
- Huon, L.-K.; Liu, S.Y.-C.; Camacho, M.; Guilleminault, C. The Association between Ophthalmologic Diseases and Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sleep Breath. 2016, 20, 1145–1154. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Villalaín, I.; Asencio, M.; García, J.; García-Rio, F. Sleep Apnea and Eye Diseases: Evidence of Association and Potential Pathogenic Mechanisms. J. Clin. Sleep Med. 2022, 18, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.L. Update on Obstructive Sleep Apnea for Neuro-Ophthalmology. Curr. Opin. Neurol. 2019, 32, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.; Fraser, C.L. Obstructive Sleep Apnea in Neuro-Ophthalmology. J. Neuroophthalmol. 2019, 39, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.K.; Park, S.J.; Byun, S.J.; Park, K.H.; Kim, J.-W.; Hwang, J.-M. Obstructive Sleep Apnoea and Increased Risk of Non-Arteritic Anterior Ischaemic Optic Neuropathy. Br. J. Ophthalmol. 2019, 103, 1123–1128. [Google Scholar] [CrossRef]
- Bialer, O.Y.; Stiebel-Kalish, H. Evaluation and Management of Nonarteritic Anterior Ischemic Optic Neuropathy: A National Survey. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 3323–3330. [Google Scholar] [CrossRef]
- Behbehani, R.; Ali, A.; Al-Moosa, A. Risk Factors and Visual Outcome of Non-Arteritic Ischemic Optic Neuropathy (NAION): Experience of a Tertiary Center in Kuwait. PLoS ONE 2021, 16, e0247126. [Google Scholar] [CrossRef]
- Salvetat, M.L.; Pellegrini, F.; Spadea, L.; Salati, C.; Zeppieri, M. Non-Arteritic Anterior Ischemic Optic Neuropathy (NA-AION): A Comprehensive Overview. Vision 2023, 7, 72. [Google Scholar] [CrossRef]
- DiCaro, M.V.; Lei, K.; Yee, B.; Tak, T. The Effects of Obstructive Sleep Apnea on the Cardiovascular System: A Comprehensive Review. J. Clin. Med. 2024, 13, 3223. [Google Scholar] [CrossRef]
- Sun, M.; Lee, C.; Liao, Y.J.; Sun, C. Nonarteritic Anterior Ischaemic Optic Neuropathy and Its Association with Obstructive Sleep Apnoea: A Health Insurance Database Study. Acta Ophthalmol. 2019, 97, e64–e70. [Google Scholar] [CrossRef]
- Chang, M.Y.; Keltner, J.L. Risk Factors for Fellow Eye Involvement in Nonarteritic Anterior Ischemic Optic Neuropathy. J. Neuroophthalmol. 2019, 39, 147–152. [Google Scholar] [CrossRef]
- Kentis, S.; Shaw, J.S.; Richey, L.N.; Young, L.; Kosyakova, N.; Bryant, B.R.; Esagoff, A.I.; Buenaver, L.F.; Salas, R.M.E.; Peters, M.E. A Systematic Review of Sleep Disturbance in Idiopathic Intracranial Hypertension. Neurol. Clin. Pract. 2025, 15, e200372. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.; Sundaram, A.N.E.; Veitch, M.; Aziz, A.; Gurges, P.; Bingeliene, A.; Tyndel, F.; Kendzerska, T.; Murray, B.J.; Boulos, M.I. Obstructive Sleep Apnea in Those with Idiopathic Intracranial Hypertension Undergoing Diagnostic In-Laboratory Polysomnography. Sleep Med. 2024, 114, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.; Berman, G.; Sumithran, P.; Mollan, S.P. Current Understanding of the Pathophysiology of Idiopathic Intracranial Hypertension. Curr. Neurol. Neurosci. Rep. 2025, 25, 31. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.J.; Lee, J.S.; Kim, C.Y.; Bae, H.W. Association between Normal Tension Glaucoma and the Risk of Obstructive Sleep Apnoea Using the STOP-Bang Questionnaire. Eye 2025, 39, 1420–1425. [Google Scholar] [CrossRef]
- Cheong, A.J.Y.; Wang, S.K.X.; Woon, C.Y.; Yap, K.H.; Ng, K.J.Y.; Xu, F.W.X.; Alkan, U.; Ng, A.C.W.; See, A.; Loh, S.R.H.; et al. Obstructive Sleep Apnoea and Glaucoma: A Systematic Review and Meta-Analysis. Eye 2023, 37, 3065–3083. [Google Scholar] [CrossRef]
- Yu, B.E.; Cheung, R.; Hutnik, C.; Malvankar-Mehta, M.S. Prevalence of Obstructive Sleep Apnea in Glaucoma Patients: A Systematic Review and Meta-Analysis. J. Curr. Glaucoma Pract. 2022, 15, 109–116. [Google Scholar] [CrossRef]
- Abdullayev, A.; Tekeli, O.; Yanık, Ö.; Acıcan, T.; Gülbay, B. Investigation of the Presence of Glaucoma in Patients with Obstructive Sleep Apnea Syndrome Using and Not Using Continuous Positive Airway Pressure Treatment. Turk. J. Ophthalmol. 2019, 49, 134–141. [Google Scholar] [CrossRef]
- Goyal, M.; Tiwari, U.S.; Jaseja, H. Pathophysiology of the Comorbidity of Glaucoma with Obstructive Sleep Apnea: A Postulation. Eur. J. Ophthalmol. 2021, 31, 2776–2780. [Google Scholar] [CrossRef]
- Tejero-Garcés, G.; Ascaso, F.J.; Casas, P.; Adiego, M.I.; Baptista, P.; O’Connor-Reina, C.; Vicente, E.; Plaza, G. Assessment of the Effectiveness of Obstructive Sleep Apnea Treatment Using Optical Coherence Tomography to Evaluate Retinal Findings. J. Clin. Med. 2022, 11, 815. [Google Scholar] [CrossRef]
- Yan, Y.R.; Zhang, L.; Lin, Y.N.; Sun, X.W.; Ding, Y.J.; Li, N.; Li, H.P.; Li, S.Q.; Zhou, J.P.; Li, Q.Y. Chronic Intermittent Hypoxia-Induced Mitochondrial Dysfunction Mediates Endothelial Injury via the TXNIP/NLRP3/IL-1β Signaling Pathway. Free Radic. Biol. Med. 2021, 165, 401–410. [Google Scholar] [CrossRef]
- Donkor, N.; Gardner, J.J.; Bradshaw, J.L.; Cunningham, R.L.; Inman, D.M. Ocular Inflammation and Oxidative Stress as a Result of Chronic Intermittent Hypoxia: A Rat Model of Sleep Apnea. Antioxidants 2024, 13, 878. [Google Scholar] [CrossRef] [PubMed]
- Al Saeed, A.A.; AlShabib, N.S.; Al Taisan, A.A.; Kreary, Y.A. Association of Retinal Vascular Manifestation and Obstructive Sleep Apnea (OSA): A Narrative Review. Clin. Ophthalmol. 2021, 15, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, T.R.; Mihălțan, F.D. Ocular Pathology Associated with Obstructive Sleep Apnea Syndrome. Romanian J. Ophthalmol. 2020, 64, 261–268. [Google Scholar] [CrossRef]
- Mentek, M.; Aptel, F.; Godin-Ribuot, D.; Tamisier, R.; Pepin, J.-L.; Chiquet, C. Diseases of the Retina and the Optic Nerve Associated with Obstructive Sleep Apnea. Sleep Med. Rev. 2018, 38, 113–130. [Google Scholar] [CrossRef]
- Kısabay Ak, A.; Batum, M.; Göktalay, T.; Mayali, H.; Kurt, E.; Selçuki, D.; Yılmaz, H. Evaluation of Retinal Fiber Thickness and Visual Pathways with Optic Coherence Tomography and Pattern Visual Evoked Potential in Different Clinical Stages of Obstructive Sleep Apnea Syndrome. Doc. Ophthalmol. Adv. Ophthalmol. 2020, 141, 33–43. [Google Scholar] [CrossRef]
- Wang, J.S.; Xie, H.T.; Jia, Y.; Zhang, M.C. Retinal Nerve Fiber Layer Thickness Changes in Obstructive Sleep Apnea Syndrome: A Systematic Review and Meta-Analysis. Int. J. Ophthalmol. 2016, 9, 1651. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, W.-B.; Zhou, M.; Jin, Y.-T.; Sun, G.-S.; Zhao, L.; Han, F.; Qu, J.-F.; Shi, X.; Zhao, M.-W. Diurnal Changes of Retinal Microvascular Circulation and RNFL Thickness Measured by Optical Coherence Tomography Angiography in Patients with Obstructive Sleep Apnea–Hypopnea. Front. Endocrinol. 2022, 13, 947586. [Google Scholar] [CrossRef]
- Christou, E.E.; Kostikas, K.; Asproudis, C.; Zafeiropoulos, P.; Stefaniotou, M.; Asproudis, I. Retinal Microcirculation Characteristics in Obstructive Sleep Apnea/Hypopnea Syndrome Evaluated by OCT-Angiography: A Literature Review. Int. Ophthalmol. 2022, 42, 3977–3991. [Google Scholar] [CrossRef]
- Venkatesh, R.; Pereira, A.; Aseem, A.; Jain, K.; Sangai, S.; Shetty, R.; Yadav, N.K. Association Between Sleep Apnea Risk Score and Retinal Microvasculature Using Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2021, 221, 55–64. [Google Scholar] [CrossRef]
- Ba-Ali, S.; Jennum, P.J.; Brøndsted, A.E.; Heegaard, S.; Lund-Andersen, H. The Role of Obstructive Sleep Apnea in Vision-Threatening Diabetic Retinopathy—A National Register-Based Study. J. Pers. Med. 2023, 13, 1529. [Google Scholar] [CrossRef]
- Morsy, N.E.; Amani, B.E.; Magda, A.A.; Nabil, A.J.; Pandi-Perumal, S.R.; BaHammam, A.S.; Spence, D.W.; Lundmark, P.O.; Zaki, N.F. Prevalence and Predictors of Ocular Complications in Obstructive Sleep Apnea Patients: A Cross-Sectional Case-Control Study. Open Respir. Med. J. 2019, 13, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Casas, P.; Ascaso, F.J.; Vicente, E.; Tejero-Garcés, G.; Adiego, M.I.; Cristóbal, J.A. Visual Field Defects and Retinal Nerve Fiber Imaging in Patients with Obstructive Sleep Apnea Syndrome and in Healthy Controls. BMC Ophthalmol. 2018, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Lee, S.K.; Kim, S.; Kim, R.E.Y.; Nam, H.R.; Siddiquee, A.T.; Thomas, R.J.; Hwang, I.; Yoon, J.-E.; Yun, C.-H.; et al. Association of Obstructive Sleep Apnea With White Matter Integrity and Cognitive Performance Over a 4-Year Period in Middle to Late Adulthood. JAMA Netw. Open 2022, 5, e2222999. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.L.; Kim, H.R.; Kim, H.; Seong, J.-K.; Joo, E.Y. White Matter Tract-Specific Alterations in Male Patients with Untreated Obstructive Sleep Apnea Are Associated with Worse Cognitive Function. Sleep 2020, 43, zsz247. [Google Scholar] [CrossRef]
- Santos, M.; Hofmann, R.J. Ocular Manifestations of Obstructive Sleep Apnea. J. Clin. Sleep Med. 2017, 13, 1345–1348. [Google Scholar] [CrossRef]
- Rostampour, M.; Noori, K.; Heidari, M.; Fadaei, R.; Tahmasian, M.; Khazaie, H.; Zarei, M. White Matter Alterations in Patients with Obstructive Sleep Apnea: A Systematic Review of Diffusion MRI Studies. Sleep Med. 2020, 75, 236–245. [Google Scholar] [CrossRef]
- Majeed, H.A.; Al-Rubiay, Y.; Abbas, A.A.; Nuaimi, M.E.A.; Khammas, H.M.; Alsaedi, Z.A.; Al Jammal, A.M.; Abdlhasn, M.M.; Abdul-Gaffar, A.M.; Mohammed, O.S.; et al. An Overview of Neuro-Ophthalmic Disorders at Jenna Ophthalmic Center, Baghdad, Iraq (2021–2022). J. Med. Life 2024, 17, 99–108. [Google Scholar] [CrossRef]
- Lee, S.S.Y.; Nilagiri, V.K.; Mackey, D.A. Sleep and Eye Disease: A Review. Clin. Experiment. Ophthalmol. 2022, 50, 334–344. [Google Scholar] [CrossRef]
- Liu, B.; Yu, Y.; Liu, W.; Deng, T.; Xiang, D. Risk Factors for Non-Arteritic Anterior Ischemic Optic Neuropathy: A Large Scale Meta-Analysis. Front. Med. 2021, 8, 618353. [Google Scholar] [CrossRef]
- Kumar, R.; Chavez, A.S.; Macey, P.M.; Woo, M.A.; Yan-Go, F.L.; Harper, R.M. Altered Global and Regional Brain Mean Diffusivity in Patients with Obstructive Sleep Apnea. J. Neurosci. Res. 2012, 90, 2043–2052. [Google Scholar] [CrossRef]
- De Terán, T.D.; Boira, I.; Cerveró, A.; Casado, A.; Lopez-de-Eguileta, A.; Fonseca, S.; Muñoz, P.; Nebot, C.; Nicolini, A.; Banfi, P.; et al. Benefit of Continuous Positive Airway Pressure on Optic Nerve Damage in Patients with Obstructive Sleep Apnea. Sleep Breath. 2025, 29, 173. [Google Scholar] [CrossRef] [PubMed]
- Kongchan, P.; Banhiran, W.; Chirapapaisan, N.; Kasemsuk, N. The Effect of Continuous Positive Airway Pressure Therapy on Intraocular Pressure in Patients with OSA: A Systematic Review and Meta-Analysis. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2025, 21, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Tribble, J.R.; Hui, F.; Quintero, H.; El Hajji, S.; Bell, K.; Di Polo, A.; Williams, P.A. Neuroprotection in Glaucoma: Mechanisms beyond Intraocular Pressure Lowering. Mol. Aspects Med. 2023, 92, 101193. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Sochal, M. Evaluation of HIF-1 Involvement in the BDNF and ProBDNF Signaling Pathways among Obstructive Sleep Apnea Patients. Int. J. Mol. Sci. 2022, 23, 14876. [Google Scholar] [CrossRef]
- Gabryelska, A.; Turkiewicz, S.; Ditmer, M.; Sochal, M. Neurotrophins in the Neuropathophysiology, Course, and Complications of Obstructive Sleep Apnea—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 1808. [Google Scholar] [CrossRef]
- Mesentier-Louro, L.A.; Shariati, M.A.; Dalal, R.; Camargo, A.; Kumar, V.; Shamskhou, E.A.; De Jesus Perez, V.; Liao, Y.J. Systemic Hypoxia Led to Little Retinal Neuronal Loss and Dramatic Optic Nerve Glial Response. Exp. Eye Res. 2020, 193, 107957. [Google Scholar] [CrossRef]
- Husain, S.; Leveckis, R. Pharmacological Regulation of HIF-1α, RGC Death, and Glaucoma. Curr. Opin. Pharmacol. 2024, 77, 102467. [Google Scholar] [CrossRef]
- Vadlapatla, R.; Vadlapudi, A.; Mitra, A. Hypoxia-Inducible Factor-1 (HIF-1): A Potential Target for Intervention in Ocular Neovascular Diseases. Curr. Drug Targets 2013, 14, 919–935. [Google Scholar] [CrossRef]
- DeMichele, E.; Buret, A.G.; Taylor, C.T. Hypoxia-Inducible Factor-Driven Glycolytic Adaptations in Host-Microbe Interactions. Pflüg. Arch.—Eur. J. Physiol. 2024, 476, 1353–1368. [Google Scholar] [CrossRef]
- Mentek, M.; Morand, J.; Baldazza, M.; Faury, G.; Aptel, F.; Pepin, J.L.; Godin-Ribuot, D.; Chiquet, C. Chronic Intermittent Hypoxia Alters Rat Ophthalmic Artery Reactivity Through Oxidative Stress, Endothelin and Endothelium-Derived Hyperpolarizing Pathways. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5256. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Selective Activation of Inflammatory Pathways by Intermittent Hypoxia in Obstructive Sleep Apnea Syndrome. Circulation 2005, 112, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Kok, L.T.; Gnoni, V.; Muza, R.; Nesbitt, A.; Leschziner, G.; Wong, S.H. Prevalence and Utility of Overnight Pulse Oximetry as a Screening Tool for Obstructive Sleep Apnoea in Newly Diagnosed Idiopathic Intracranial Hypertension. Eye 2023, 37, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Riedel, C.S.; Martinez-Tejada, I.; Andresen, M.; Wilhjelm, J.E.; Jennum, P.; Juhler, M. Transient Intracranial Pressure Elevations (B Waves) Are Associated with Sleep Apnea. Fluids Barriers CNS 2023, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Purvin, V.A. Papilledema and Obstructive Sleep Apnea Syndrome. Arch. Ophthalmol. 2000, 118, 1626. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Guan, J.; Yi, H.; Yin, S. Effect of CPAP on Endothelial Function in Subjects With Obstructive Sleep Apnea: A Meta-Analysis. Respir. Care 2015, 60, 749–755. [Google Scholar] [CrossRef]
- Lavalle, S.; Masiello, E.; Iannella, G.; Magliulo, G.; Pace, A.; Lechien, J.R.; Calvo-Henriquez, C.; Cocuzza, S.; Parisi, F.M.; Favier, V.; et al. Unraveling the Complexities of Oxidative Stress and Inflammation Biomarkers in Obstructive Sleep Apnea Syndrome: A Comprehensive Review. Life 2024, 14, 425. [Google Scholar] [CrossRef]
- Yamada, E.; Himori, N.; Kunikata, H.; Omodaka, K.; Ogawa, H.; Ichinose, M.; Nakazawa, T. The Relationship between Increased Oxidative Stress and Visual Field Defect Progression in Glaucoma Patients with Sleep Apnoea Syndrome. Acta Ophthalmol. 2018, 96, e479–e484. [Google Scholar] [CrossRef]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. Oxidative Stress Markers among Obstructive Sleep Apnea Patients. Oxid. Med. Cell. Longev. 2021, 2021, 9681595. [Google Scholar] [CrossRef]
- Casini, G.; Ola, M.S.; Koulen, P. Editorial: Neurodegeneration and Neuroprotection in Retinal Disease, Volume II. Front. Neurosci. 2022, 16, 1009228. [Google Scholar] [CrossRef]
- Chang, J.L.; Goldberg, A.N.; Alt, J.A.; Mohammed, A.; Ashbrook, L.; Auckley, D.; Ayappa, I.; Bakhtiar, H.; Barrera, J.E.; Bartley, B.L.; et al. International Consensus Statement on Obstructive Sleep Apnea. Int. Forum Allergy Rhinol. 2023, 13, 1061–1482. [Google Scholar] [CrossRef]
- Meliante, P.G.; Zoccali, F.; Cascone, F.; Di Stefano, V.; Greco, A.; de Vincentiis, M.; Petrella, C.; Fiore, M.; Minni, A.; Barbato, C. Molecular Pathology, Oxidative Stress, and Biomarkers in Obstructive Sleep Apnea. Int. J. Mol. Sci. 2023, 13, 5478. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M.; Grunstein, R.R. Clinical Side Effects of Continuous Positive Airway Pressure in Patients with Obstructive Sleep Apnoea. Respirology 2020, 25, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Tsai, M.-S.; Lee, L.-A.; Hsin, L.-J.; Lee, Y.-C.; Lin, W.-N.; Lu, Y.-A.; Shen, S.-C.; Cheng, W.-N.; Chaing, Y.-T. Palatal Hybrid Surgery for Obstructive Sleep Apnea-State-of-the-Art Annotation of Uvulopalatopharyngoplasty. Biomed. J. 2023, 46, 100568. [Google Scholar] [CrossRef] [PubMed]
- Martinovic, D.; Tokic, D.; Puizina-Mladinic, E.; Kadic, S.; Lesin, A.; Lupi-Ferandin, S.; Kumric, M.; Bozic, J. Oromaxillofacial Surgery: Both a Treatment and a Possible Cause of Obstructive Sleep Apnea—A Narrative Review. Life 2023, 13, 142. [Google Scholar] [CrossRef]
- Francis, C.E.; Quinnell, T. Mandibular Advancement Devices for OSA: An Alternative to CPAP? Pulm. Ther. 2021, 7, 25–36. [Google Scholar] [CrossRef]
- Malhotra, A.; Heilmann, C.R.; Banerjee, K.K.; Dunn, J.P.; Bunck, M.C.; Bednarik, J. Weight Reduction and the Impact on Apnea-Hypopnea Index: A Systematic Meta-Analysis. Sleep Med. 2024, 121, 26–31. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; Li, G.; Liu, P. Pharmacological Interventions for the Treatment of Obstructive Sleep Apnea Syndrome. Front. Med. 2024, 11, 1359461. [Google Scholar] [CrossRef]
- Hoy, S.M. Solriamfetol: A Review in Excessive Daytime Sleepiness Associated with Narcolepsy and Obstructive Sleep Apnoea. CNS Drugs 2023, 37, 1009–1020. [Google Scholar] [CrossRef]
- Anderer, S. FDA Approves Tirzepatide as First Drug for Obstructive Sleep Apnea. JAMA 2025, 333, 656. [Google Scholar] [CrossRef]
- Boppana, T.K.; Mittal, S.; Madan, K.; Tiwari, P.; Mohan, A.; Hadda, V. Antioxidant Therapies for Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sleep Breath. Schlaf Atm. 2024, 28, 1513–1522. [Google Scholar] [CrossRef]
- Jeppesen, K.; Good, A.R.; Dyrhaug, I.D.; Johansen, M.B.; Primdahl, J. Patients’ Experiences of Barriers and Facilitators with Continuous Positive Airway Pressure Therapy in Obstructive Sleep Apnoea—A Qualitative Interview Study. Arch. Public Health Arch. Belg. Sante Publique 2025, 83, 157. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Pepin, J.-L.; Cistulli, P.A.; Wimms, A.; Lavergne, F.; Sert Kuniyoshi, F.H.; Munson, S.H.; Schuler, B.; Reddy Badikol, S.; Wolfe, K.C.; et al. Positive Airway Pressure Therapy and All-cause and Cardiovascular Mortality in People with Obstructive Sleep Apnoea: A Systematic Review and Meta-Analysis of Randomised Controlled Trials and Confounder-Adjusted, Non-Randomised Controlled Studies. Lancet Respir. Med. 2025, 13, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.L.; Sankari, A.; Sharma, S. Continuous Positive Airway Pressure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Gharib, A. Effect of Continuous Positive Airway Pressure on the Respiratory System: A Comprehensive Review. Egypt. J. Bronchol. 2023, 17, 1. [Google Scholar] [CrossRef]
- Khazaie, S.; Mehra, R.; Bhambra, R.; Moul, D.E.; Foldvary-Schaefer, N.; Vanek, R.; Bena, J.; Morrison, S.; Walia, H.K. Impact of a Multidisciplinary Sleep Apnea Management Group Clinic on Positive Airway Pressure Adherence and Patient-Reported Outcomes: A Randomized Controlled Trial. Sleep Breath. 2025, 29, 149. [Google Scholar] [CrossRef]
- Taweesedt, P.; Najeeb, H.; Surani, S. Patient-Centered Therapy for Obstructive Sleep Apnea: A Review. Medicina (Mex.) 2022, 58, 1338. [Google Scholar] [CrossRef]
- Jaganathan, N.; Kwon, Y.; Healy, W.J.; Taskar, V. The Emerging Role of Pharmacotherapy in Obstructive Sleep Apnea. J. Otorhinolaryngol. Hear. Balance Med. 2024, 5, 12. [Google Scholar] [CrossRef]
- Kim, J.-W.; Won, T.-B.; Rhee, C.-S.; Park, Y.M.; Yoon, I.-Y.; Cho, S.-W. Polysomnographic Phenotyping of Obstructive Sleep Apnea and Its Implications in Mortality in Korea. Sci. Rep. 2020, 10, 13207. [Google Scholar] [CrossRef]
- Subramani, Y.; Singh, M.; Wong, J.; Kushida, C.A.; Malhotra, A.; Chung, F. Understanding Phenotypes of Obstructive Sleep Apnea: Applications in Anesthesia, Surgery, and Perioperative Medicine. Anesth. Analg. 2017, 124, 179–191. [Google Scholar] [CrossRef]
- Gasa, M.; Salord, N.; Fontanilles, E.; Pérez Ramos, S.; Prado, E.; Pallarés, N.; Santos Pérez, S.; Monasterio, C. Polysomnographic Phenotypes of Obstructive Sleep Apnea in a Real-Life Cohort: A Pathophysiological Approach. Arch. Bronconeumol. 2023, 59, 638–644. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, L.-M.; Lou, H.; Cheng, J.-W.; Wei, R.-L. The Association Between Obstructive Sleep Apnea and Nonarteritic Anterior Ischemic Optic Neuropathy: A Systematic Review and Meta-Analysis. Curr. Eye Res. 2016, 41, 987–992. [Google Scholar] [CrossRef]
- Simpson, P.J.L.; Hoyos, C.M.; Celermajer, D.; Liu, P.Y.; Ng, M.K.C. Effects of Continuous Positive Airway Pressure on Endothelial Function and Circulating Progenitor Cells in Obstructive Sleep Apnoea: A Randomised Sham-Controlled Study. Int. J. Cardiol. 2013, 168, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Al-Halawani, M.; Kyung, C.; Liang, F.; Kaplan, I.; Moon, J.; Clerger, G.; Sabin, B.; Barnes, A.; Al-Ajam, M. Treatment of Obstructive Sleep Apnea with CPAP Improves Chronic Inflammation Measured by Neutrophil-to-Lymphocyte Ratio. J. Clin. Sleep Med. 2020, 16, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Placidi, F.; Palmieri, M.G.; Izzi, F.; Ludovisi, R.; Mercuri, N.B.; Pierantozzi, M. Continuous Positive Airway Pressure Treatment May Improve Optic Nerve Function in Obstructive Sleep Apnea: An Electrophysiological Study. J. Clin. Sleep Med. 2018, 14, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.; Title, M.; Spurr, K.; Morrison, D. Positive Airway Pressure Therapy Adherence and Outcomes in Obstructive Sleep Apnea: An Exploratory Longitudinal Retrospective Randomized Chart Review. Can. J. Respir. Ther. 2024, 60, 28. [Google Scholar] [CrossRef]
- Li, Z.; Cai, S.; Wang, J.; Chen, R. Predictors of the Efficacy for Daytime Sleepiness in Patients With Obstructive Sleep Apnea With Continual Positive Airway Pressure Therapy: A Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2022, 13, 911996. [Google Scholar] [CrossRef]
- Chai-Coetzer, C.L.; Luo, Y.-M.; Antic, N.A.; Zhang, X.-L.; Chen, B.-Y.; He, Q.-Y.; Heeley, E.; Huang, S.-G.; Anderson, C.; Zhong, N.-S.; et al. Predictors of Long-Term Adherence to Continuous Positive Airway Pressure Therapy in Patients with Obstructive Sleep Apnea and Cardiovascular Disease in the SAVE Study. Sleep 2013, 36, 1929–1937. [Google Scholar] [CrossRef]
- Lal, C.; Ayappa, I.; Ayas, N.; Beaudin, A.E.; Hoyos, C.; Kushida, C.A.; Kaminska, M.; Mullins, A.; Naismith, S.L.; Osorio, R.S.; et al. The Link between Obstructive Sleep Apnea and Neurocognitive Impairment: An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2022, 19, 1245–1256. [Google Scholar] [CrossRef]
- Iannella, G.; Pace, A.; Bellizzi, M.G.; Magliulo, G.; Greco, A.; De Virgilio, A.; Croce, E.; Gioacchini, F.M.; Re, M.; Costantino, A.; et al. The Global Burden of Obstructive Sleep Apnea. Diagnostics 2025, 15, 1088. [Google Scholar] [CrossRef]
- Ciuntu, R.E.; Anton, N.; Cantemir, A.; Alexa, A.I.; Bogdanici, C.M.; Boişteanu, D.; Vasilescu, A.; Danielescu, C.; Ghiga, G.; Ciuntu, B.M.; et al. Clinical Study on the Ocular Manifestations in Patients with Obstructive Sleep Apnea Syndrome—Preliminary Results. Appl. Sci. 2021, 11, 569. [Google Scholar] [CrossRef]
- Bussan, K.A.; Stuard, W.L.; Mussi, N.; Lee, W.; Whitson, J.T.; Issioui, Y.; Rowe, A.A.; Wert, K.J.; Robertson, D.M. Differential Effects of Obstructive Sleep Apnea on the Corneal Subbasal Nerve Plexus and Retinal Nerve Fiber Layer. PLoS ONE 2022, 17, e0266483. [Google Scholar] [CrossRef]
- Ucak, T.; Unver, E. Alterations in Parafoveal and Optic Disc Vessel Densities in Patients with Obstructive Sleep Apnea Syndrome. J. Ophthalmol. 2020, 2020, 4034382. [Google Scholar] [CrossRef] [PubMed]
- Duong-Quy, S.; Nguyen-Huu, H.; Hoang-Chau-Bao, D.; Tran-Duc, S.; Nguyen-Thi-Hong, L.; Nguyen-Duy, T.; Tang-Thi-Thao, T.; Phan, C.; Bui-Diem, K.; Vu-Tran-Thien, Q.; et al. Personalized Medicine and Obstructive Sleep Apnea. J. Pers. Med. 2022, 12, 2034. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.A.; Redline, S.; Sands, S.A.; Owens, R.L. More Than the Sum of the Respiratory Events: Personalized Medicine Approaches for Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2019, 200, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Mokhlesi, B.; Ham, S.A.; Gozal, D. The Effect of Sex and Age on the Comorbidity Burden of OSA: An Observational Analysis from a Large Nationwide US Health Claims Database. Eur. Respir. J. 2016, 47, 1162–1169. [Google Scholar] [CrossRef]
- Bublitz, M.; Adra, N.; Hijazi, L.; Shaib, F.; Attarian, H.; Bourjeily, G. A Narrative Review of Sex and Gender Differences in Sleep Disordered Breathing: Gaps and Opportunities. Life 2022, 12, 2003. [Google Scholar] [CrossRef]
| OSA Severity | AHI (Events/Hour) | Clinical Interpretation |
|---|---|---|
| Mild | 5–15 | May present with mild daytime sleepiness |
| Moderate | 15–30 | Increased risk for systemic and ocular complications |
| Severe | >30 | High risk for cardiovascular, neurological, and ocular damage |
| Ophthalmological Disorder | Pathophysiological Mechanisms | Main Clinical Features | Type of Association with OSA | References |
|---|---|---|---|---|
| Non-arteritic anterior ischaemic optic neuropathy | Nocturnal hypoxia, impaired optic nerve perfusion, autoregulatory dysfunction | Sudden, painless vision loss; altitudinal visual field defects | Strong epidemiological and biological association | [12,41,42,43,44,45,46,47,48,49,50,51] |
| Papilledema (especially in IIH) | Increased intracranial pressure during REM sleep, hypercapnia, venous outflow resistance | Transient visual obscurations, headache, bilateral disc swelling | Suggested association, particularly in obese women with IIH | [7,13,52,53,54] |
| Glaucoma (especially normal-tension glaucoma) | Oxidative stress, endothelial dysfunction, impaired ocular perfusion, mitochondrial damage | Progressive visual field loss, optic nerve cupping; NTG with normal IOP | Strong clinical and pathophysiological association | [7,12,41,42,55,56,57,58,59,60,61,62] |
| Retinal microvascular changes | IH-induced vascular dysregulation, VEGF overexpression, endothelial dysfunction | Reduced vessel density (using OCTA), RNFL thinning, choroidal thickening | Growing evidence, particularly in severe OSA | [17,42,63,64,65,66,67,68,69,70] |
| Diabetic retinopathy (in OSA patients with DM) | Enhanced VEGF and inflammation, oxidative stress, disrupted autoregulation | Haemorrhages, neovascularisation, macular oedema | Exacerbation of disease severity and progression | [7,12,14,17,71,72] |
| Visual field defects | Subclinical RNFL damage, cortical hypoxia | Arcuate or peripheral defects, generalised depression | Subclinical or early-stage manifestation in OSA patients | [12,14,42,43,72,73,74] |
| Oculomotor dysfunction and visual processing deficits | Hypoxic injury to cranial nerves and visual cortex | Diplopia, convergence insufficiency, delayed visual reaction, impaired contrast sensitivity | Frequently under-recognised, linked to cognitive dysfunction | [12,13,42,74,75,76,77] |
| Mechanism of Association | Key Molecular Mediators | Mode of Action and Effect | Ocular Consequences | References |
|---|---|---|---|---|
| Intermittent hypoxia | HIF-1α, HIF-2α, VEGF, ROS | Induces oxidative stress, mitochondrial dysfunction, angiogenesis, and apoptotic pathways | Retinal ganglion cell loss, neovascularisation, oedema | [12,13,14,16,87,88,89,90,91,92] |
| Intracranial pressure fluctuations | CO2, cerebral vasodilation mediators, venous pressure | Elevates ICP, disrupts axoplasmic flow, impairs optic nerve perfusion | Papilledema, optic disc oedema, visual obscurations | [12,13,14,93,94,95] |
| Vascular dysregulation | ET-1, nitric oxide, eNOS, COX-2 | Causes endothelial dysfunction, vasoconstriction, and impaired ocular blood flow | Glaucoma progression, optic nerve ischemia | [15,16,22,41,65,67,89,96] |
| Systemic and local inflammation | TNF-α, IL-6, IL-8, CRP, NF-κB, ICAM-1, VCAM-1 | Promotes leukocyte adhesion, increases vascular permeability, disrupts the blood–retinal barrier | Retinal inflammation and microangiopathy, optic nerve inflammation, gliosis | [13,14,21,42,44,65,67,87,88,89,91,92,97] |
| Oxidative stress | ROS, mitochondrial damage, depleted antioxidants | Triggers cellular apoptosis, reduces antioxidant defences, impairs neurovascular homeostasis | Retinal ganglion cell apoptosis, RNFL thinning, glaucomatous optic neuropathy | [18,19,20,38,59,62,91,97,98,99] |
| Neurodegeneration | Microglial activation, glutamate excitotoxicity, BDNF depletion | Leads to axonal injury, synaptic loss, and impaired neurotrophic support | Visual processing deficits, optic nerve degeneration | [12,13,14,84,85,86,98,99,100] |
| Extracellular matrix remodelling | MMPs, collagen turnover enzymes | Alters ECM integrity, weakens connective tissues | Floppy eyelid syndrome, predisposition to exfoliative glaucoma | [14,19,32,42] |
| Therapeutic Options | Targeted Mechanisms | Neuro-Ophthalmological Benefits | Limitations | References |
|---|---|---|---|---|
| Continuous positive airway pressure | Maintains upper airway patency, reduces IH and ICP, improves optic nerve perfusion | Improves retinal and optic nerve oxygenation, slows glaucoma progression, stabilises ocular vasculature | Poor adherence in some patients may cause ocular dryness or an increase in ICP at high pressure levels | [2,23,36,96,103,112,113,114,115,116,117] |
| Surgical interventions (UPPP, maxillomandibular advancement) | Improve airway patency and reduce OSA severity | Alternative for patients non-compliant with CPAP; may significantly reduce AHI | Invasive procedures with surgical risk; variable long-term efficacy | [2,23,103,104,105,106,114] |
| Pharmacological agents (solriamfetol, modafinil) | Promote wakefulness via dopamine and norepinephrine reuptake inhibition; may indirectly reduce oxidative stress | Enhance alertness and quality of life in CPAP-intolerant patients | Do not directly treat neuro-ophthalmological complications; risk of cardiovascular side effects | [2,23,108,109,110] |
| Weight loss and lifestyle modification | Decreases upper airway resistance, reduces systemic inflammation, and oxidative stress | Lowers AHI and systemic disease burden; improves visual and general health outcomes | Requires sustained patient motivation; effects may vary based on comorbidities | [2,23,107] |
| Antioxidants and neuroprotective agents | Reduce oxidative stress, preserve mitochondrial integrity, reduce inflammation, support ganglion cell survival | Potential to protect retinal ganglion cells and optic nerve from hypoxic injury, may preserve visual function | Limited clinical trial evidence; effects may be modest | [102,108,111,118] |
| Interdisciplinary care (sleep medicine, ophthalmology, neurology) | Provides integrated management and monitoring | Optimises visual and neurological outcomes | Requires structured care coordination; not always feasible in all settings | [7,14,43,101,102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaštelan, S.; Kozina, L.; Alaber, M.; Tomić, Z.; Andrešić, M.; Bakija, I.; Bućan, D.; Matejić, T.; Vidović, D. Neuro-Ophthalmological Disorders Associated with Obstructive Sleep Apnoea. Int. J. Mol. Sci. 2025, 26, 6649. https://doi.org/10.3390/ijms26146649
Kaštelan S, Kozina L, Alaber M, Tomić Z, Andrešić M, Bakija I, Bućan D, Matejić T, Vidović D. Neuro-Ophthalmological Disorders Associated with Obstructive Sleep Apnoea. International Journal of Molecular Sciences. 2025; 26(14):6649. https://doi.org/10.3390/ijms26146649
Chicago/Turabian StyleKaštelan, Snježana, Lea Kozina, Maja Alaber, Zora Tomić, Marina Andrešić, Ivana Bakija, Diana Bućan, Tomislav Matejić, and Domagoj Vidović. 2025. "Neuro-Ophthalmological Disorders Associated with Obstructive Sleep Apnoea" International Journal of Molecular Sciences 26, no. 14: 6649. https://doi.org/10.3390/ijms26146649
APA StyleKaštelan, S., Kozina, L., Alaber, M., Tomić, Z., Andrešić, M., Bakija, I., Bućan, D., Matejić, T., & Vidović, D. (2025). Neuro-Ophthalmological Disorders Associated with Obstructive Sleep Apnoea. International Journal of Molecular Sciences, 26(14), 6649. https://doi.org/10.3390/ijms26146649







