Drought Stress in Roses: A Comprehensive Review of Morphophysiological, Biochemical, and Molecular Responses
Abstract
1. Introduction
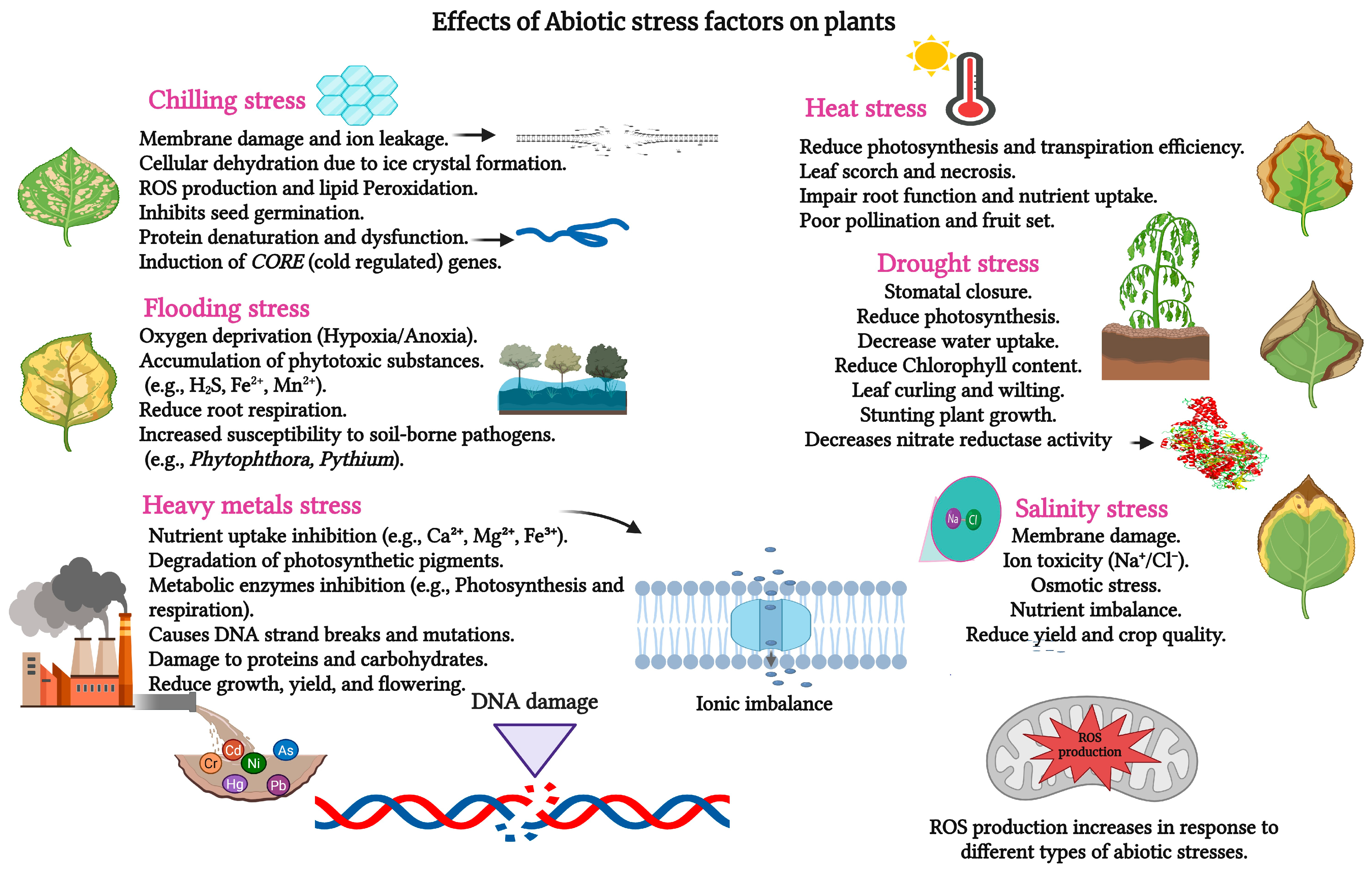
2. Plant DS: Causes and Classification
2.1. Causes of Water Deficiency in Plants
2.2. Classification of DS
3. Effect of DS on the Morphology, Physiology, and Biochemistry of Roses
3.1. Drought-Morphological Attributes in Roses
3.2. Physiological and Biochemical Responses
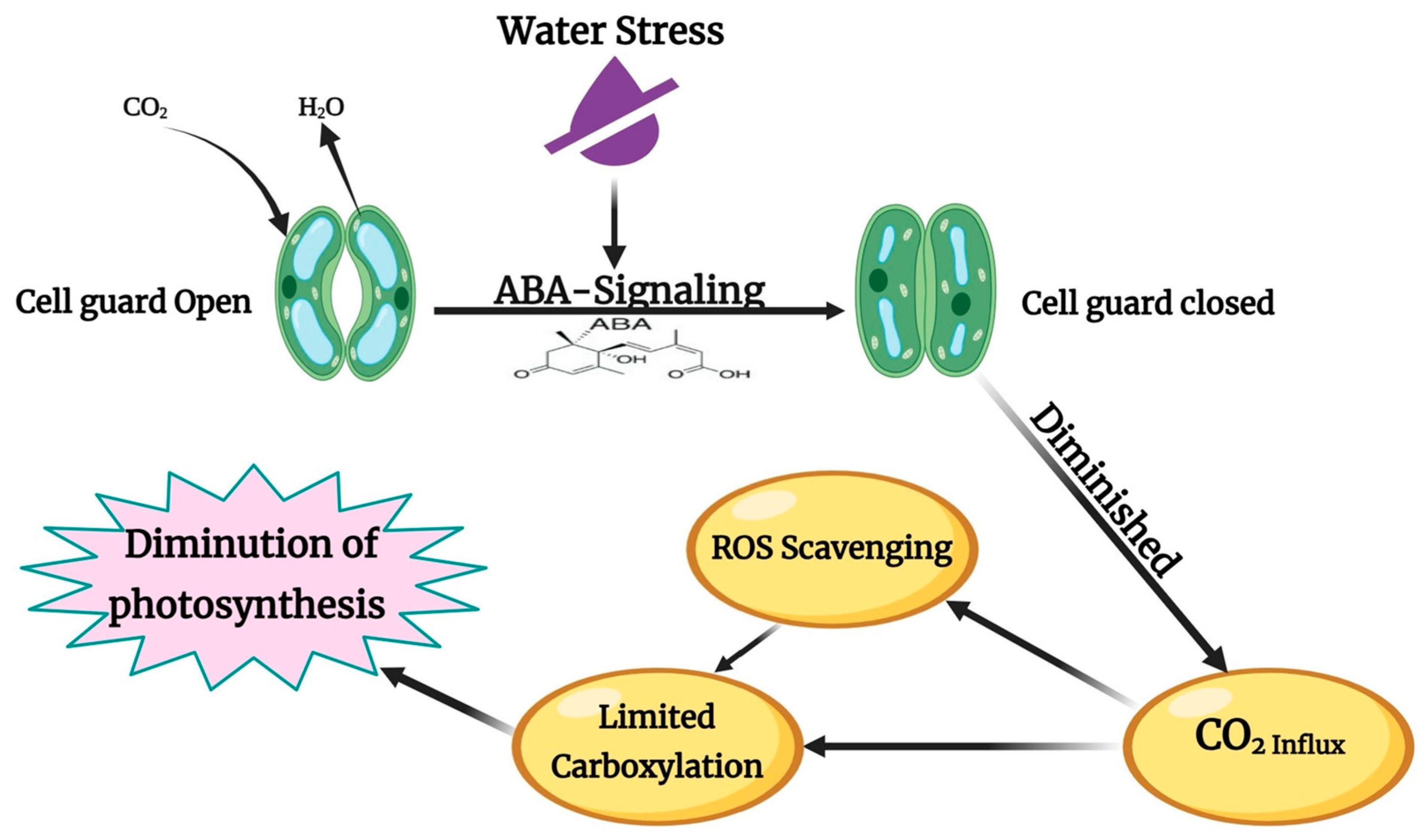
3.2.1. Gaseous Exchange
3.2.2. Photosynthesis Parameters: Chlorophyll Content and Photosynthesis
3.2.3. Water-Relations
3.2.4. Nutrient Relations
3.2.5. Phytohormone Regulation
3.2.6. Oxidative Stress: Production of Reactive Oxygen Species and Adaptive Responses
3.2.7. Osmotic Adjustment (OA)
3.2.8. Influence of DS on Rose Petal Essential Oil Yield and Composition
4. Influence of Dehydration Stress on Rose Quality and Postharvest Longevity
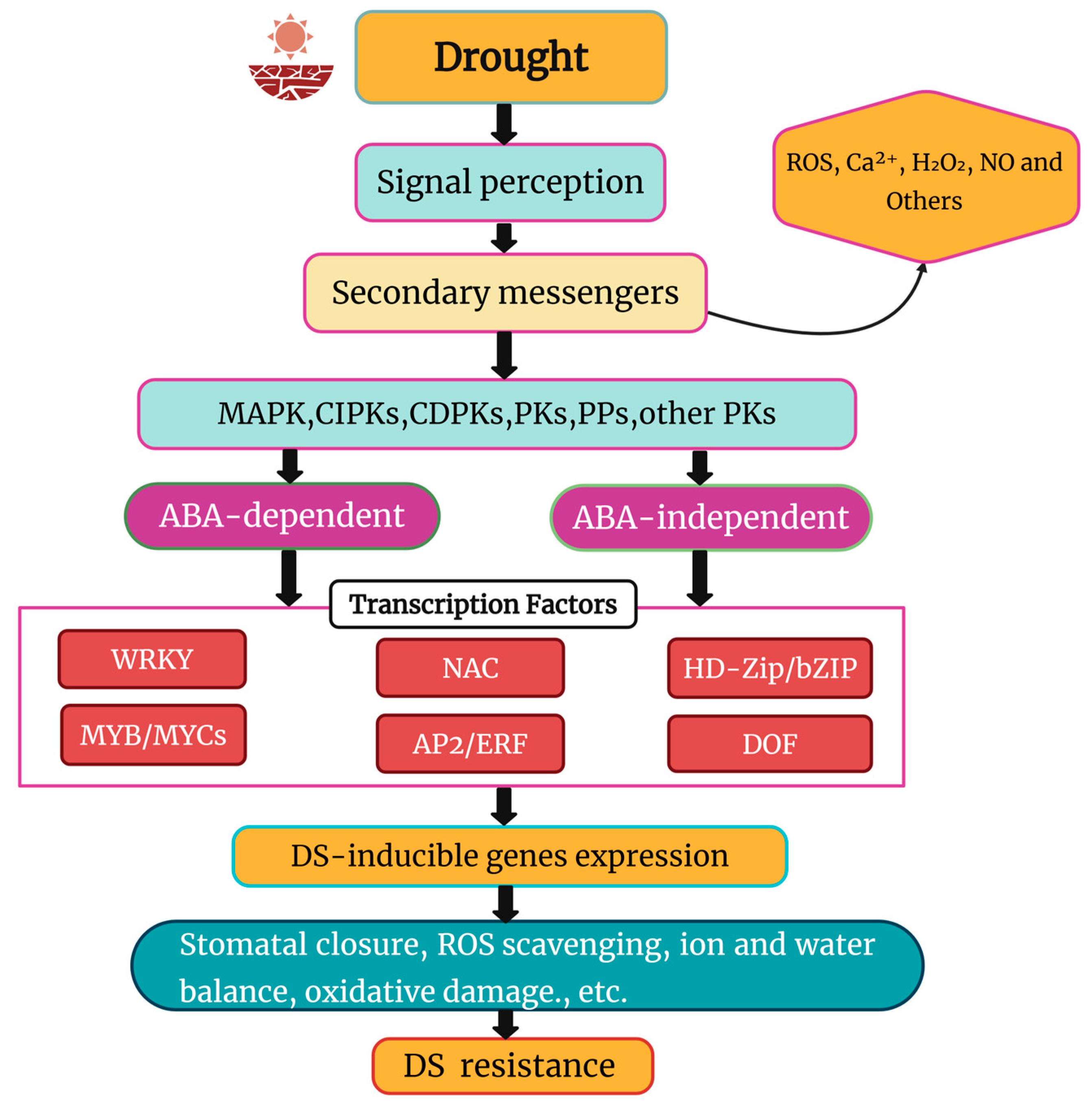
5. Molecular Responses to Drought Stress
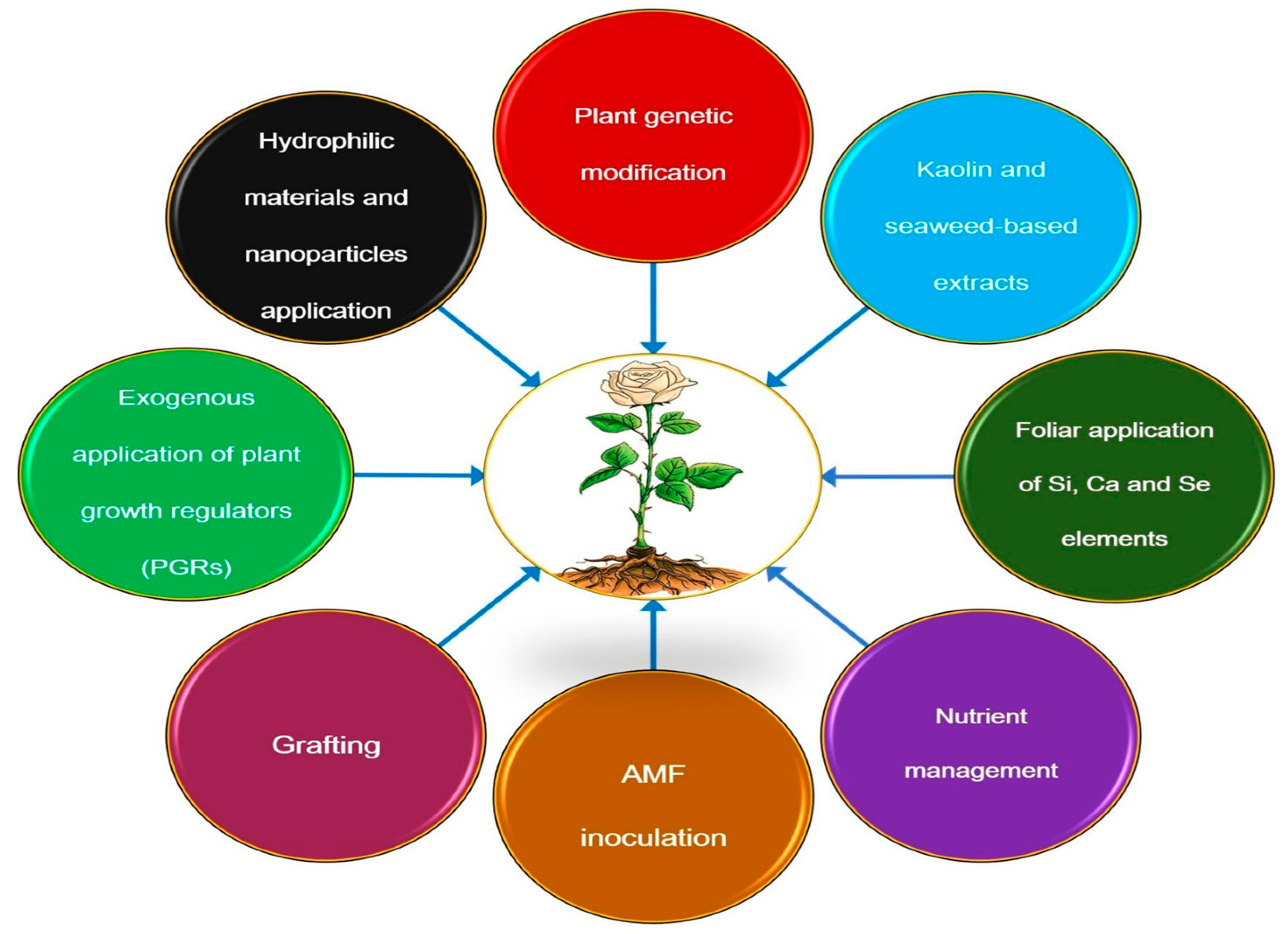
6. Different Strategies to Mitigate the Adverse Effects of Drought Stress on Rose Plants
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.; Fischhoff, D.A.; Hodges, C.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D.; et al. Radically rethinking agriculture for the 21st century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Ghosh, A.; Li, Z.-G.; Siddiqui, M.N.; Fujita, M.; Tran, L.-S.P. Methylglyoxal—A signaling molecule in plant abiotic stress responses. Free. Radic. Biol. Med. 2018, 122, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N. Alleviation of drought stress on rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015, 197, 373–380. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Ma, Z.; Boken, V.K.; Zeng, H.; Shang, J.; Igor, S.; Wang, J.; Yan, N. Regional differences in the performance of drought mitigation measures in 12 major wheat-growing regions of the world. Agric. Water Manag. 2022, 273, 107888. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- De Vries, D.; Dubois, L.A. Rose breeding: Past, present, prospects. Acta Hortic. 1996, 424, 241–248. [Google Scholar] [CrossRef]
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Leus, L.; Van Laere, K.; De Riek, J.; Van Huylenbroeck, J. Ornamental crops. In Handbook of Plant Breeding; Huylenbroeck, J.V., Ed.; Springer: Cham, Switzerland, 2018; pp. 719–767. [Google Scholar]
- Che, D.; Zhang, X.; Zhang, J.; Yang, T.; Zhang, W.; Xiong, Y. Research progress on locus location of quantitative traits in Rosa plants. J. Acta Hortic. Sin. 2016, 43, 1765–1775. [Google Scholar]
- Mordor Intelligence. Netherlands Floriculture Market—Growth, Trends, COVID-19 Impact, and Forecasts (2021–2026); Mordor Intelligence: Hyderabad, India, 2020. [Google Scholar]
- DataIntelo. Rose Market Report: Global Forecast From 2025 To 2033. Available online: https://dataintelo.com/report/global-rose-market (accessed on 18 April 2025).
- OEC. Roses (HS: 060311). Available online: https://oec.world/en/profile/hs/roses (accessed on 18 April 2025).
- Cai, X.; Niu, G.; Starman, T.; Hall, C. Response of six garden roses (Rosa × hybrida L.) to salt stress. Sci. Hortic. 2014, 168, 27–32. [Google Scholar] [CrossRef]
- Erbas, S.; Baydar, H. Variation in scent compounds of oil-bearing rose (Rosa damascena Mill.) produced by headspace solid phase microextraction, hydrodistillation and solvent extraction. Rec. Nat. Prod. 2016, 10, 555. [Google Scholar]
- Raymond, O. Domestication et Sélection Dirigée chez le Rosier: Analyse Historique via les Phénotypes Morphologique, Chimique et Biochimique. Ph.D. Thesis, Université Claude Bernard Lyon 1, Lyon, France, 1999. [Google Scholar]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Su, L.; Wang, Y.; Geng, Z.; Lin, S.; Zhang, Y.; Yu, S.; Fu, L.; Liu, Q.; Cheng, C.; et al. Role of RcTINY2 in the Regulation of Drought and Salt Stress Response in Arabidopsis and Rose. Horticulturae 2022, 8, 747. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of abiotic stress on crops. In Sustainable Crop Production; IntechOpen: London, UK, 2020; Volume 3, pp. 5–16. [Google Scholar]
- Saini, H.S.; Westgate, M.E. Reproductive development in grain crops during drought. Adv. Agron. 1999, 68, 59–96. [Google Scholar]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Change 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic Adjustment and Plant Adaptation to Drought Stress. In Drought Stress Tolerance in Plants, Vol 1: Physiology and Biochemistry; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 105–143. [Google Scholar]
- Malinowska, M.; Donnison, I.; Robson, P. Morphological and physiological traits that explain yield response to drought stress in Miscanthus. Agronomy 2020, 10, 1194. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, N.; Armada, E.; Duque, E.; Roldán, A.; Azcón, R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: Effectiveness of autochthonous or allochthonous strains. J. Plant Physiol. 2015, 174, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-fertilization as an emerging fertilization technique: Why can modern agriculture benefit from its use? Plants 2020, 10, 2. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Mishra, S.S.; Behera, P.K.; Panda, D. Genotypic variability for drought tolerance-related morpho-physiological traits among indigenous rice landraces of Jeypore tract of Odisha, India. J. Crop Improv. 2019, 33, 254–278. [Google Scholar] [CrossRef]
- Panda, D.; Mishra, S.S.; Behera, P.K. Drought tolerance in rice: Focus on recent mechanisms and approaches. Rice Sci. 2021, 28, 119–132. [Google Scholar] [CrossRef]
- Hessini, K.; Wasli, H.; Al-Yasi, H.M.; Ali, E.F.; Issa, A.A.; Hassan, F.A.S.; Siddique, K.H.M. Graded Moisture Deficit Effect on Secondary Metabolites, Antioxidant, and Inhibitory Enzyme Activities in Leaf Extracts of Rosa damascena Mill. var. trigentipetala. Horticulturae 2022, 8, 177. [Google Scholar] [CrossRef]
- Abdel-Salam, E.; Alatar, A.; El-Sheikh, M.A. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. 2018, 25, 1772–1780. [Google Scholar] [CrossRef]
- Seyed Hajizadeh, H.; Azizi, S.; Rasouli, F.; Kaya, O. Evaluation of nano-silicon efficiency on compatible solutes and nutrient status of Damask rose affected by in vitro simulated drought stress. Chem. Biol. Technol. Agric. 2023, 10, 22. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.H.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef]
- Aalam, F.; Rezaei Nejad, A.; Mousavi-Fard, S.; Raji, M.; Nikoloudakis, N.; Goumenaki, E.; Fanourakis, D. Water Deficit Severity during the Preceding Year Determines Plant Tolerance to Subsequent Year Drought Stress Challenges: A Case Study in Damask Rose. Horticulturae 2024, 10, 462. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Z.; Kim, W. Effect of drought stress on shoot growth and physiological response in the cut rose ‘Charming Black’at different developmental stages. Hortic. Environ. Biotechnol. 2019, 60, 1–8. [Google Scholar] [CrossRef]
- Williams, M.H.; Rosenqvist, E.; Buchhave, M. Response of potted miniature roses (Rosa × hybrida) to reduced water availability during production. J. Hortic. Sci. Biotechnol. 1999, 74, 301–308. [Google Scholar] [CrossRef]
- Dolatkhahi, A.; Shoor, M.; Bannayan, M.; Tehranifar, A.; Alizadeh, A. Water deficit decreases gas exchange parameters and marketable quality of Rosa hybrida ‘Club-Nika’irrespective of training systems. J. Agric. Sci. Technol. 2020, 22, 837–849. [Google Scholar]
- Li, W.; Fu, L.; Geng, Z.; Zhao, X.; Liu, Q.; Jiang, X. Physiological characteristic changes and full-length transcriptome of rose (Rosa chinensis) roots and leaves in response to drought stress. Plant Cell Physiol. 2020, 61, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Farahani, H.; Sajedi, N.A.; Madani, H.; Changizi, M.; Naeini, M.R. Effect of foliar-applied silicon on flower yield and essential oil composition of damask rose (Rosa damascena Miller) under water deficit stress. Silicon 2021, 13, 4463–4472. [Google Scholar] [CrossRef]
- Katsoulas, N.; Kittas, C.; Dimokas, G.; Lykas, C. Effect of irrigation frequency on rose flower production and quality. Biosyst. Eng. 2006, 93, 237–244. [Google Scholar] [CrossRef]
- Fascella, G.; Gugliuzza, G.; Mammano, M.; Maggiore, P. Effect of different irrigation regimes on yield and quality of hydroponic cut roses. In Proceedings of the VI International Symposium on Rose Research and Cultivation, Hannover, Germany, 25 August 2013; Volume 1064, pp. 259–263. [Google Scholar]
- Raviv, M.; Blom, T.J. The effect of water availability and quality on photosynthesis and productivity of soilless-grown cut roses. Sci. Hortic. 2001, 88, 257–276. [Google Scholar] [CrossRef]
- Sotelo-Cuitiva, Y.M.; Restrepo-Díaz, H.; García-Castro, A.; Ramírez-Godoy, A.; Flórez-Roncancio, V.J. Effect of kaolin film particle applications (Surround WP®) and water deficit on physiological characteristics in rose cut plants (Rose spp L.). Am. J. Plant Sci 2011, 2, 354–358. [Google Scholar] [CrossRef]
- Farahani, H.; Sajedi, N.; Madani, H.; Changizi, M.; Naeini, M.R. Effect of potassium silicate on water use efficiency, quantitative traits and essential oil yield of damask rose (Rosa damascena Miller) under water deficit stress. Iran. J. Hortic. Sci. 2021, 52, 171–182. [Google Scholar]
- Nedkov, N.; Matev, A.; Ovcharova, A. “Additional yield–irrigation depth” relationship for white bearing rose (Rosa alba L.). Ovidius Univ. Ann. Ser. Civ. Eng. 2014, 16, 91–104. [Google Scholar]
- Ober, E.S.; Alahmad, S.; Cockram, J.; Forestan, C.; Hickey, L.T.; Kant, J.; Maccaferri, M.; Marr, E.; Milner, M.; Pinto, F.; et al. Wheat root systems as a breeding target for climate resilience. Theor. Appl. Genet. 2021, 134, 1645–1662. [Google Scholar] [CrossRef]
- Amtmann, A.; Bennett, M.J.; Henry, A. Root phenotypes for the future. Plant Cell Environ. 2022, 45, 595–601. [Google Scholar] [CrossRef]
- Meister, A.; Finger, S.; Hause, G.; Blume, A. Morphological changes of bacterial model membrane vesicles. Eur. J. Lipid Sci. Technol. 2014, 116, 1228–1233. [Google Scholar] [CrossRef]
- Ashfaq, W.; Brodie, G.; Fuentes, S.; Pang, A.; Gupta, D. Silicon improves root system and canopy physiology in wheat under drought stress. Plant Soil 2024, 502, 279–296. [Google Scholar] [CrossRef]
- McWilliams, D. Drought Strategies for Cotton; Cooperative Extension Service Circular 582; College of Agriculture and Home Economics; New Mexico State University: Las Cruces, NM, USA, 2003; pp. 1–5. [Google Scholar]
- Hessini, K.; Kronzucker, H.J.; Abdelly, C.; Cruz, C. Drought stress obliterates the preference for ammonium as an N source in the C4 plant Spartina alterniflora. J. Plant Physiol. 2017, 213, 98–107. [Google Scholar] [CrossRef]
- Niu, G.; Rodriguez, D.S. Growth and physiological responses of four rose rootstocks to drought stress. J. Am. Soc. Hortic. Sci. 2009, 134, 202–209. [Google Scholar] [CrossRef]
- Murphy, J.E.; Burns, J.H. Rosa multiflora’s performance under water stress: The role of positive and negative density-dependent intraspecific interactions. Plant Ecol. 2019, 220, 951–963. [Google Scholar] [CrossRef]
- Bolla, A.; Voyiatzis, D.; Koukourikou-Petridou, M.; Chimonidou, D. Photosynthetic parameters and cut-flower yield of rose ‘Eurored’(HT) are adversely affected by mild water stress irrespective of substrate composition. Sci. Hortic. 2010, 126, 390–394. [Google Scholar] [CrossRef]
- Cai, X.; Starman, T.; Niu, G.; Hall, C.; Lombardini, L. Response of Selected Garden Roses to Drought Stress. HortScience 2012, 47, 1050–1055. [Google Scholar] [CrossRef]
- Harp, D.A.; Kay, K.; Zlesak, D.C.; George, S. The Effect of Rose Root Size on Drought Stress Tolerance and Landscape Plant Performance. Tex. J. Agric. Nat. Resour. 2015, 28, 82–88. [Google Scholar]
- Zhao, X.; Lin, S.; Yu, S.; Zhang, Y.; Su, L.; Geng, L.; Cheng, C.; Jiang, X. Exogenous calcium enhances the physiological status and photosynthetic capacity of rose under drought stress. Hortic. Plant J. 2024, 10, 853–865. [Google Scholar] [CrossRef]
- Jia, X.; Feng, H.; Bu, Y.; Ji, N.; Lyu, Y.; Zhao, S. Comparative transcriptome and weighted gene co-expression network analysis identify key transcription factors of Rosa chinensis ‘Old Blush’after exposure to a gradual drought stress followed by recovery. Front. Genet. 2021, 12, 690264. [Google Scholar] [CrossRef]
- Blum, A. Crop responses to drought and the interpretation of adaptation. Plant Growth Regul. 1996, 20, 135–148. [Google Scholar] [CrossRef]
- Li, L.; Zhu, H.; Ju, Y.; Lv, Z.; Qian, C.; Zhang, C.; Lu, Y.; Wang, J.; Li, W. Comparison of microstructure and physiological response of the leaves of six Rosa rugosa genotypes under drought stress. Ornam. Plant Res. 2024, 4, e016. [Google Scholar] [CrossRef]
- Sher, A.; Khan, A.; Hussain, S.; Cai, L.J.; Ahmad, M.I.; Jamro, S.A.; Rashid, A. Significance of chemical priming on yield and yield components of wheat under drought stress. Am. J. Plant Sci. 2017, 8, 1339–1344. [Google Scholar] [CrossRef]
- Sarwar, J.M.; Nozulaidi, B.N.M.; Khairi, B.C.L.M.; Mohd, K.Y. Effects of water stress on rice production: Bioavailability of potassium in soil. J. Stress Physiol. Biochem. 2013, 9, 97–107. [Google Scholar]
- Zhu, R.; Wu, F.; Zhou, S.; Hu, T.; Huang, J.; Gao, Y. Cumulative effects of drought–flood abrupt alternation on the photosynthetic characteristics of rice. Environ. Exp. Bot. 2020, 169, 103–901. [Google Scholar] [CrossRef]
- Yokota, A.; Kawasaki, S.; Iwano, M.; Nakamura, C.; Miyake, C.; Akashi, K. Citrulline and DRIP-1 protein (ArgE homologue) in drought tolerance of wild watermelon. Ann. Bot. 2002, 89, 825–832. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, L.; Ma, F.; Zou, Y.; Liang, D. Growth, biomass allocation, and water use efficiency of 31 apple cultivars grown under two water regimes. Agrofor. Syst. 2012, 84, 117–129. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M. Plant drought stress: Effects, mechanisms and management. Sustain. Agric. 2009, 23, 153–188. [Google Scholar]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H. Comparison of selected biochemical characteristics of damask rose and dog rose under deficit irrigation conditions. Italus Hortus 2022, 29, 138–155. [Google Scholar] [CrossRef]
- Li, G.; Xu, W.; Jing, P.; Hou, X.; Fan, X. Overexpression of VyDOF8, a Chinese wild grapevine transcription factor gene, enhances drought tolerance in transgenic tobacco. Environ. Exp. Bot. 2021, 190, 104592. [Google Scholar] [CrossRef]
- Shi, L.; Kim, W.S. Shoot growth and physiological disorder of cut rose ‘Charming Black’as affected by drought stress during nocturnal supplemental lighting. Hortic. Environ. Biotechnol. 2014, 55, 91–96. [Google Scholar] [CrossRef]
- Gadzinowska, J.; Hura, K.; Ostrowska, A.; Hura, T. Activity of the photosynthetic apparatus in dehydrated leaves of a perennial shrub Rosa rubiginosa L. with different levels of drought memory. Environ. Exp. Bot. 2021, 187, 104493. [Google Scholar] [CrossRef]
- Kirkham, M.B. Principles of Soil and Plant Water Relations, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef]
- Alavi, S.M.; Kamali, M.; Selahvarzi, Y.; Ansari, S. Deficit irrigation strategies (PRD, SDI) and titanium nanoparticles improve water use efficiency and flower quality in greenhouse-grown cut roses. Sci. Rep. 2023, 13, 18019. [Google Scholar] [CrossRef]
- De Dauw, K.; Van Labeke, M.-C.; Leus, L.; Van Huylenbroeck, J. Drought tolerance screening of a Rosa population. In Proceedings of the II International Symposium on Woody Ornamentals of the Temperate Zone, Ghent, Belgium, 1–4 July 2012; Volume 990, pp. 121–127. [Google Scholar]
- Fascella, G.; Agnello, S.; Maggiore, P.; Zizzo, G.; Guarino, L. Effect of Controlled Irrigation Methods Using Climatic Parameters on Yield and Quality of Hydroponic Cut Roses. Acta Hortic. 2010, 870, 65–72. [Google Scholar] [CrossRef]
- Shangguan, Z.; Shao, M.; Dyckmans, J. Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J. Plant Physiol. 2000, 156, 46–51. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Hu, Y.; Burucs, Z.; von Tucher, S.; Schmidhalter, U. Short-term effects of drought and salinity on mineral nutrient distribution along growing leaves of maize seedlings. Environ. Exp. Bot. 2007, 60, 268–275. [Google Scholar] [CrossRef]
- Tommasini, L.; Svensson, J.T.; Rodriguez, E.M.; Wahid, A.; Malatrasi, M.; Kato, K.; Wanamaker, S.; Resnik, J.; Close, T.J. Dehydrin gene expression provides an indicator of low temperature and drought stress: Transcriptome-based analysis of barley (Hordeum vulgare L.). Funct. Integr. Genom. 2008, 8, 387–405. [Google Scholar] [CrossRef]
- Kuchenbuch, R.; Claassen, N.; Jungk, A. Potassium availability in relation to soil moisture: I. Effect of soil moisture on potassium diffusion, root growth and potassium uptake of onion plants/Kaliumverfügbarkeit in Beziehung zur Bodenfeuchte: I. Wirkung des Wassergehaltes auf die K-Diffusion, das Wurzelwachstum. Plant Soil 1986, 95, 221–231. [Google Scholar]
- Martínez-Viveros, O.; Jorquera, M.A.; Crowley, D.; Gajardo, G.; Mora, M. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Chattha, M.U.; Ullah, M.A.; Sulaman, S.; Nawaz, M.; Zhiqiang, W.; Yanqin, M.; Guoqin, H. The role of potassium in plants under drought stress: Mini review. J. Basic Appl. Sci. 2017, 13, 268–271. [Google Scholar] [CrossRef]
- Luo, D.; Li, J.; Luo, J.; Ma, Y.; Wang, Y.; Liu, W.; Rodriguez, L.G.; Yao, Y. Responses to solar UV-B exclusion and drought stress in two cultivars of chestnut rose with different leaf thickness. Forests 2022, 14, 50. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Kharal, M.A. Role of phytohormones in stress tolerance of plants. In Plant, Soil and Microbes: Volume 2: Mechanisms and Molecular Interactions; Hakeem, K.R., Akhtar, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 385–421. [Google Scholar]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, K.; Hou, X.; Li, H.; Wang, G.; Xu, Z.; Xiong, A. Transcriptome profiling reveals the association of multiple genes and pathways contributing to hormonal control in celery leaves. Acta Biochim. Biophy. Sin. 2019, 51, 524–534. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Ismail; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Lee, I.-J. Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. BioMed Res. Int. 2018, 2018, 7696831. [Google Scholar] [CrossRef] [PubMed]
- Ciura, J.; Kruk, J. Phytohormones as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2018, 229, 32–40. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Gnanasekaran, P.; Kumari, P.; Lakra, N.; Lal, S.K.; Pawar, J.; Narayan, O.P. An overview of recent advancement in phytohormones-mediated stress management and drought tolerance in crop plants. Plant Gene 2021, 25, 100264. [Google Scholar]
- Lee, M.; Jung, J.-H.; Han, D.-Y.; Seo, P.J.; Park, W.J.; Park, C.-M. Activation of a flavin monooxygenase gene YUCCA7 enhances drought resistance in Arabidopsis. Planta 2012, 235, 923–938. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Zhang, W.; Yan, S.; Wang, R.; Zhao, J.; Li, Y.; Qi, Z.; Sun, Z.; Zhu, Z. The putative auxin efflux carrier OsPIN3t is involved in the drought stress response and drought tolerance. Plant J. 2012, 72, 805–816. [Google Scholar] [CrossRef]
- Nir, I.; Moshelion, M.; Weiss, D. The Arabidopsis GIBBERELLIN METHYL TRANSFERASE 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant Cell Environ. 2014, 37, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Shi, H.; Shang, X.; Zhao, Z.; Fang, Y.; Wu, H.; Luo, P.; Cui, Y.; Chen, W. RhMED15a-like, a subunit of the Mediator complex, is involved in the drought stress response in Rosa hybrida. BMC Plant Biol. 2024, 24, 351. [Google Scholar] [CrossRef]
- Gadzinowska, J.; Ostrowska, A.; Hura, K.; Dziurka, M.; Pawłowska, B.; Hura, T. Physiological traits determining high adaptation potential of sweet briar (Rosa rubiginosa L.) at early stage of growth to dry lands. Sci. Rep. 2019, 9, 19390. [Google Scholar] [CrossRef] [PubMed]
- Shahbani, Z.; Kosh-Khui, M.; Salehi, H.; Kafi, M.; Kamgar Haghighi, A.A.; Eshghi, S.; Omidi, M. Hormonal and Physiological Changes in Miniature Roses (Rosa chinensis Jacq. var. minima Rehd.) Exposed to Water Deficit and Salinity Stress Conditions. Gesunde Pflanz. 2023, 75, 1781–1797. [Google Scholar]
- Zhang, F.; Wang, P.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Arch. Agron. Soil Sci. 2019, 65, 1316–1330. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Strzelak, A.; Ratajczak, A.; Adamiec, A.; Feleszko, W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: A mechanistic review. Int. J. Environ. Res. Public Health 2018, 15, 1033. [Google Scholar] [CrossRef]
- De Gara, L.; Foyer, C.H. Ying and Yang interplay between reactive oxygen and reactive nitrogen species controls cell functions. Plant Cell Environ. 2017, 40, 459–461. [Google Scholar] [CrossRef]
- Verma, G.; Srivastava, D.; Tiwari, P.; Chakrabarty, D. ROS modulation in crop plants under drought stress. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 311–336. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Fujita, M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (‘Triticum aestivum’ L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci. 2012, 6, 1314–1323. [Google Scholar]
- Xu, Y.; Burgess, P.; Zhang, X.; Huang, B. Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in Agrostis stolonifera. J. Exp. Bot. 2016, 67, 1979–1992. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Mellacheruvu, S.; Tamirisa, S.; Vudem, D.R.; Khareedu, V.R. Pigeonpea hybrid-proline-rich protein (CcHyPRP) confers biotic and abiotic stress tolerance in transgenic rice. Front. Plant Sci. 2016, 6, 1167. [Google Scholar]
- Tang, Y.; Bao, X.; Zhi, Y.; Wu, Q.; Guo, Y.; Yin, X.; Zeng, L.; Li, J.; Zhang, J.; He, W.; et al. Overexpression of a MYB family gene, OsMYB6, increases drought and salinity stress tolerance in transgenic rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar]
- Niu, Y.; Wang, Y.; Li, P.; Zhang, F.; Liu, H.; Zheng, G. Drought stress induces oxidative stress and the antioxidant defense system in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. Acta Physiol. Plant. 2013, 35, 1189–1200. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Y.; Yu, S.; Huang, Y.; Liu, H.; Chen, W.; He, X. Effects of drought stress on growth, physiology and secondary metabolites of Two Adonis species in Northeast China. Sci. Hortic. 2020, 259, 108795. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Lee, D.-J.; Cheema, S.A.; Aziz, T. DROUGHT STRESS: Comparative Time Course Action of the Foliar Applied Glycinebetaine, Salicylic Acid, Nitrous Oxide, Brassinosteroids and Spermine in Improving Drought Resistance of Rice. J. Agron. Crop Sci. 2010, 196, 336–345. [Google Scholar]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H.; Razi, H.; Karami, A.; Moghadam, A. Role of genes and metabolites involved in polyamines synthesis pathways and nitric oxide synthase in stomatal closure on Rosa damascena Mill. under drought stress. Plant Physiol. Biochem. 2020, 148, 53–61. [Google Scholar] [CrossRef]
- Jin, J.; Shan, N.; Ma, N.; Bai, J.; Gao, J. Regulation of ascorbate peroxidase at the transcript level is involved in tolerance to postharvest water deficit stress in the cut rose (Rosa hybrida L.) cv. Samantha. Postharvest Biol. Technol. 2006, 40, 236–243. [Google Scholar] [CrossRef]
- Jiang, Y.; Khan, M.A.; Wang, Z.; Liu, J.; Xue, J.; Gao, J.; Zhang, C. Cu/ZnSOD involved in tolerance to dehydration in cut rose (Rosa hybrida). Postharvest Biol. Technol. 2015, 100, 187–195. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Ahmad, A.; Siddiqi, T.; Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2013, 35, 1039–1050. [Google Scholar] [CrossRef]
- Mishra, S.S.; Panda, D. Leaf traits and antioxidant defense for drought tolerance during early growth stage in some popular traditional rice landraces from Koraput, India. Rice Sci. 2017, 24, 207–217. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Zlatev, Z.; Lidon, F.C. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir. J. Food Agric. (EJFA) 2012, 24. [Google Scholar]
- Kumar, S.; Sachdeva, S.; Bhat, K.; Vats, S. Plant responses to drought stress: Physiological, biochemical and molecular basis. In Biotic and Abiotic Stress Tolerance in Plants; Springer: Singapore, 2018; pp. 1–25. [Google Scholar]
- Xiang, Z.; Xu, G.; Jiang, W. Drought stress on physiological and biochemical processes in four spiny plant species. J. Zhejiang AF Univ. 2007, 24, 7. [Google Scholar]
- Grebennikova, O.; Pilkevich, R.; Gubanova, T.; Plugatar, S. Physiological and biochemical parameters of drought tolerance of some genotypes of garden roses. In BIO Web of Conferences, Proceedings of the International Scientific and Practical Conference "VAVILOV READINGS-2023" (VVRD 2023), Online, 25–26 May 2023; EDP Sciences: Les Ulis, France, 2023; p. 02014. [Google Scholar]
- Meena, Y.K.; Kaur, N. Towards an understanding of physiological and biochemical mechanisms of drought tolerance in plant. Annu. Res. Rev. Biol. 2019, 31, 1–13. [Google Scholar] [CrossRef]
- Morgan, P. Effects of abiotic stresses on plant hormone systems. In Stress Responses in Plants: Adaptation Mechanisms; Alscher, R.G., Cumming, J., Eds.; Wiley-Liss, Inc.: New York, NY, USA, 1990; pp. 313–314. [Google Scholar]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Marín-de la Rosa, N.; Lin, C.W.; Kang, Y.J.; Dhondt, S.; Gonzalez, N.; Inzé, D.; Falter-Braun, P. Drought resistance is mediated by divergent strategies in closely related Brassicaceae. New Phytol. 2019, 223, 783–797. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nam, N.H.; Chauhan, Y.S.; Johansen, C. Osmotic adjustment, water relations and carbohydrate remobilization in pigeonpea under water deficits. J. Plant Physiol. 2000, 157, 651–659. [Google Scholar] [CrossRef]
- Martìnez, J.-P.; Lutts, S.; Schanck, A.; Bajji, M.; Kinet, J.-M. Is osmotic adjustment required for water stress resistance in the Mediterranean shrub Atriplex halimus L? J. Plant Physiol. 2004, 161, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Ferchichi, S.; Hessini, K.; Dell’Aversana, E.; D’Amelia, L.; Woodrow, P.; Ciarmiello, L.F.; Fuggi, A.; Carillo, P. Hordeum vulgare and Hordeum maritimum respond to extended salinity stress displaying different temporal accumulation pattern of metabolites. Funct. Plant Biol. 2018, 45, 1096–1109. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Andrew Riseman, A.; Charlotte Jensen, C.; Michelle Williams, M. Stomatal conductivity and osmotic adjustment during acclimation to multiple cycles of drought stress in potted miniature rose (Rosa × hybrida). J. Hortic. Sci. Biotechnol. 2001, 76, 138–144. [Google Scholar] [CrossRef]
- Yang, S.; Chen, K.; Wang, S.; Gong, M. Osmoregulation as a key factor in drought hardening-induced drought tolerance in Jatropha curcas. Biol. Plant. 2015, 59, 529–536. [Google Scholar] [CrossRef]
- Surówka, E.; Hura, T. Osmoprotectants and Nonenzymatic Antioxidants in Halophytes. In Handbook of Halophytes: From Molecules to Ecosystems Towards Biosaline Agriculture; Grigore, M.-N., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1901–1930. [Google Scholar]
- Pal, P.K. Evaluation, genetic diversity, recent development of distillation method, challenges and opportunities of Rosa damascena: A review. J. Essent. Oil Bear. Plants 2013, 16, 1–10. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, A.K.; Yadav, A.K. Volatile constituents of essential oil and rose water of damask rose (Rosa damascena Mill.) cultivars from North Indian hills. Nat. Prod. Res. 2011, 25, 1577–1584. [Google Scholar] [CrossRef]
- Singh, S. Rose Oil Market Research Report Information By Application, By Product Type, By Distribution Channel, By Extraction Method, And By Region—Market Forecast Till 2032. Available online: https://www.marketresearchfuture.com/reports/rose-oil-market-7243 (accessed on 18 April 2025).
- Moein, M.; Ghasemia, Y.; Karami, F.; Tavallali, H. Composition of the Essential Oil of Rosa damascena Mill. from South of Iran: Composition of the essential oil of Rosa damascenea. Iran. J. Pharm. Sci. 2010, 6, 59–62. [Google Scholar]
- Farooqi, A.H.A.; Sharma, S. Effect of growth retardants on flowering of Rosa damascena Mill. In Proceedings of the International Congress of Plant Physiology, New Dehli, India, 15–20 February 1988; Volume 2, pp. 1369–1372. [Google Scholar]
- Kumar, R.; Sharma, S.K.; Sood, S.; Agnihotri, V.K.; Singh, B. Effect of diurnal variability and storage conditions on essential oil content and quality of damask rose (Rosa damascena Mill.) flowers in north western Himalayas. Sci. Hortic. 2013, 154, 102–108. [Google Scholar] [CrossRef]
- Ucar, Y.; Kazaz, S.; Eraslan, F.; Baydar, H. Effects of different irrigation water and nitrogen levels on the water use, rose flower yield and oil yield of Rosa damascena. Agric. Water Manag. 2017, 182, 94–102. [Google Scholar] [CrossRef]
- Ames, G.R.; Matthews, W.S.A. The distillation of essential oils. Trop. Sci. 1968, 10, 136–148. [Google Scholar]
- Yousefi, B. Screening of Rosa damascena Mill. landraces for flower yield and essential oil content in cold climates. Folia Hortic. 2016, 28, 31–40. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Rezaei Nejad, A. The influence of nitrogen-fertilizer and harvest time on the productivity of Thymus vulgaris L. Int. J. Hortic. Sci. 2000, 6, 43–46. [Google Scholar] [CrossRef]
- Kiymaz, S.; Altun, B.; Ertek, A. Effect of different water regimes and nitrogen applications on the growth, yield, essential oil content, and quality parameters of the oil rose (Rosa damascena Mill.). J. Plant Nutr. 2022, 45, 2108–2122. [Google Scholar] [CrossRef]
- Uçar, Y.; Kazaz, S.; Eraslan İnal, F.; Baydar, H.; Erbaş, S. Response of Rose (Rosa damascena Mill.) Oil Components to Different Irrigation Water and Nitrogen Applications. Ziraat Fakültesi Derg. 2024, 19, 128–140. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Y.; Wu, H.; Zhang, S.; Yang, R.; Liu, X. RhRab5ip, a new interactor of RhPIP1;1, was involved in flower opening of cut rose during water deficit. Plant Physiol. Biochem. 2022, 181, 61–70. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Miller, W.B. Nonrefrigerated dry storage can have negative effects on postharvest quality of cut Lilium. HortScience 2022, 57, 1475–1479. [Google Scholar] [CrossRef]
- Liu, D.; Liu, X.; Meng, Y.; Sun, C.; Tang, H.; Jiang, Y.; Khan, M.A.; Xue, J.; Ma, N.; Gao, J. An organ-specific role for ethylene in rose petal expansion during dehydration and rehydration. J. Exp. Bot. 2013, 64, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Woltering, E.J.; Paillart, M.J. Effect of cold storage on stomatal functionality, water relations and flower performance in cut roses. Postharvest Biol. Technol. 2018, 136, 66–73. [Google Scholar] [CrossRef]
- Ha, S.T.; Lim, J.-H.; In, B.-C. Extension of the vase life of cut roses by both improving water relations and repressing ethylene responses. Hortic. Sci. Technol. 2019, 37, 65–77. [Google Scholar] [CrossRef]
- Sukpitak, C.; Seraypheap, K.; Muñoz, P.; Munné-Bosch, S. Influence of water deficit on the longevity of ethylene-sensitive and ethylene-insensitive flowers. Environ. Exp. Bot. 2024, 219, 105647. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, C.; Jiang, X.; Kang, M.; Yin, X.; Lü, P.; Zhang, X.; Zheng, Y.; Gao, J. RhNAC2 and RhEXPA4 are involved in the regulation of dehydration tolerance during the expansion of rose petals. Plant Physiol. 2012, 160, 2064–2082. [Google Scholar] [CrossRef]
- Chu, X.; Wang, C.; Chen, X.; Lu, W.; Li, H.; Wang, X.; Hao, L.; Guo, X. The cotton WRKY gene GhWRKY41 positively regulates salt and drought stress tolerance in transgenic Nicotiana benthamiana. PLoS ONE 2015, 10, e0143022. [Google Scholar] [CrossRef]
- Khazaei, H.; Street, K.; Bari, A.; Mackay, M.; Stoddard, F.L. The FIGS (Focused Identification of Germplasm Strategy) approach identifies traits related to drought adaptation in Vicia faba genetic resources. PLoS ONE 2013, 8, e63107. [Google Scholar] [CrossRef]
- Singh, B.; Bohra, A.; Mishra, S.; Joshi, R.; Pandey, S. Embracing new-generation ‘omics’ tools to improve drought tolerance in cereal and food-legume crops. Biol. Plant. 2015, 59, 413–428. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef]
- Pérez-Clemente, R.M.; Vives, V.; Zandalinas, S.I.; López-Climent, M.F.; Muñoz, V.; Gómez-Cadenas, A. Biotechnological approaches to study plant responses to stress. BioMed Res. Int. 2013, 2013, 654120. [Google Scholar] [CrossRef]
- Liu, J.-H.; Peng, T.; Dai, W. Critical cis-acting elements and interacting transcription factors: Key players associated with abiotic stress responses in plants. Plant Mol. Biol. Rep. 2014, 32, 303–317. [Google Scholar] [CrossRef]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Huang, G.-T.; Ma, S.-L.; Bai, L.-P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.-F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef]
- Danquah, A.; De Zélicourt, A.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Mizoi, J.; Ohori, T.; Moriwaki, T.; Kidokoro, S.; Todaka, D.; Maruyama, K.; Kusakabe, K.; Osakabe, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. GmDREB2A;2, a canonical DEHYDRATION-RESPONSIVE ELEMENT-BINDING PROTEIN2-type transcription factor in soybean, is posttranslationally regulated and mediates dehydration-responsive element-dependent gene expression. Plant Physiol. 2013, 161, 346–361. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Hou, X. Genome-wide analysis of the AP2/ERF transcription factor superfamily in Chinese cabbage (Brassica rapa ssp. pekinensis). BMC Genom. 2013, 14, 573. [Google Scholar] [CrossRef]
- Anbazhagan, K.; Bhatnagar-Mathur, P.; Vadez, V.; Dumbala, S.R.; Kishor, P.K.; Sharma, K.K. DREB1A overexpression in transgenic chickpea alters key traits influencing plant water budget across water regimes. Plant Cell Rep. 2015, 34, 199–210. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Khan, S.A.; Li, M.Z.; Wang, S.M.; Yin, H.J. Revisiting the Role of Plant Transcription Factors in the Battle against Abiotic Stress. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, J.; Li, H.; Yang, C.; Zhang, C.; Zhang, X.; Khurram, Z.; Zhang, Y.; Wang, T.; Fei, Z.; et al. Transcriptional profiles of drought-responsive genes in modulating transcription signal transduction, and biochemical pathways in tomato. J. Exp. Bot. 2010, 61, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Lindemose, S.; O’Shea, C.; Jensen, M.K.; Skriver, K. Structure, function and networks of transcription factors involved in abiotic stress responses. Int. J. Mol. Sci. 2013, 14, 5842–5878. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; Kjaersgaard, T.; Nielsen, M.M.; Galberg, P.; Petersen, K.; O’shea, C.; Skriver, K. The Arabidopsis thaliana NAC transcription factor family: Structure–function relationships and determinants of ANAC019 stress signalling. Biochem. J. 2010, 426, 183–196. [Google Scholar] [CrossRef]
- Olsen, A.N.; Ernst, H.A.; Leggio, L.L.; Skriver, K. NAC transcription factors: Structurally distinct, functionally diverse. Trends Plant Sci. 2005, 10, 79–87. [Google Scholar] [CrossRef]
- Nakashima, K.; Takasaki, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. NAC transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2012, 1819, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Puranik, S.; Sahu, P.P.; Srivastava, P.S.; Prasad, M. NAC proteins: Regulation and role in stress tolerance. Trends Plant Sci. 2012, 17, 369–381. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Li, M.; Yan, Y.; Liu, X.; Li, L. Dual function of NAC072 in ABF3-mediated ABA-responsive gene regulation in Arabidopsis. Front. Plant Sci. 2016, 7, 1075. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, C.; Lü, P.; Jiang, G.; Liu, X.; Dai, F.; Gao, J. Rh NAC 3, a stress-associated NAC transcription factor, has a role in dehydration tolerance through regulating osmotic stress-related genes in rose petals. Plant Biotechnol. J. 2014, 12, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Li, S.; Li, W.; Hao, Q.; Wan, X.; Wang, K.; Liu, Q.; Liu, Q.; Jiang, X. RhNAC31, a novel rose NAC transcription factor, enhances tolerance to multiple abiotic stresses in Arabidopsis. Acta Physiol. Plant. 2019, 41, 1–16. [Google Scholar] [CrossRef]
- Geng, L.; Yu, S.; Zhang, Y.; Su, L.; Lu, W.; Zhu, H.; Jiang, X. Transcription factor RcNAC091 enhances rose drought tolerance through the abscisic acid–dependent pathway. Plant Physiol. 2023, 193, 1695–1712. [Google Scholar] [CrossRef]
- Fujita, M.; Fujita, Y.; Maruyama, K.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Tran, L.S.P.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 2004, 39, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, Z.; Wang, H.; Zhao, X.; Su, L.; Geng, L.; Lu, Y.; Tong, B.; Liu, Q.; Jiang, X. Genome-wide analysis of BURP genes and identification of a BURP-V gene RcBURP4 in Rosa chinensis. Plant Cell Rep. 2022, 41, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Agarwal, P.K.; Joshi, A.J.; Sopory, S.K.; Reddy, M.K. Overexpression of PgDREB2A transcription factor enhances abiotic stress tolerance and activates downstream stress-responsive genes. Mol. Biol. Rep. 2010, 37, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Du, C.; Hu, K.; Xian, S.; Liu, C.; Fan, J.; Tu, J.; Fu, T. Dynamic transcriptome analysis reveals AP2/ERF transcription factors responsible for cold stress in rapeseed (Brassica napus L.). Mol. Genet. Genom. 2016, 291, 1053–1067. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.; Zou, J.; Fang, Y.; Wang, L.; Wang, M.; Jiang, X.; Liu, Y.; Gao, J.; Zhang, C. A RhABF2/Ferritin module affects rose (Rosa hybrida) petal dehydration tolerance and senescence by modulating iron levels. Plant J. 2017, 92, 1157–1169. [Google Scholar] [CrossRef]
- Ma, J.; Li, M.-Y.; Wang, F.; Tang, J.; Xiong, A.-S. Genome-wide analysis of Dof family transcription factors and their responses to abiotic stresses in Chinese cabbage. BMC Genom. 2015, 16, 33. [Google Scholar] [CrossRef]
- Chen, P.; Yan, M.; Li, L.; He, J.; Zhou, S.; Li, Z.; Niu, C.; Bao, C.; Zhi, F.; Ma, F.; et al. The apple DNA-binding one zinc-finger protein MdDof54 promotes drought resistance. Hortic. Res. 2020, 7, 195. [Google Scholar] [CrossRef]
- Cao, L.; Ye, F.; Fahim, A.M.; Ma, C.; Pang, Y.; Zhang, X.; Zhang, Q.; Lu, X. Transcription factor ZmDof22 enhances drought tolerance by regulating stomatal movement and antioxidant enzymes activities in maize (Zea mays L.). Theor. Appl. Genet. 2024, 137, 132. [Google Scholar] [CrossRef]
- Zhao, C.; Bai, H.; Li, C.; Pang, Z.; Xuan, L.; Lv, D.; Niu, S. Genome-Wide Identification of the DOF Gene Family in Kiwifruit (Actinidia chinensis) and Functional Validation of AcDOF22 in Response to Drought Stress. Int. J. Mol. Sci. 2024, 25, 9103. [Google Scholar] [CrossRef]
- Nan, H.; Ludlow, R.A.; Lu, M.; An, H. Genome-wide analysis of Dof genes and their response to abiotic stress in rose (Rosa chinensis). Front. Genet. 2021, 12, 538733. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Osbourn, A.; Ma, P. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Xie, N.; Li, Y.; Zhao, Z.; Luo, P.; Cui, Y.; Rao, X.; Chen, W. Mediator Subunit RhMED15a Regulates Drought Tolerance in Rose. Horticulturae 2024, 10, 84. [Google Scholar] [CrossRef]
- Hou, Y.; Fan, C.; Sun, J.; Chang, Y.; Lu, J.; Sun, J.; Wang, C.; Liu, J. Genome-wide Identification, evolution, and expression analysis of the TCP gene family in rose (Rosa chinensis Jacq.). Horticulturae 2022, 8, 961. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cheon, K.-S.; Kim, S.Y.; Kim, J.H.; Kwon, O.H.; Lee, H.J.; Kim, W.H. Expression of SOD2 enhances tolerance to drought stress in roses. Hortic. Environ. Biotechnol. 2020, 61, 569–576. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.; Zou, J.; Zhang, X.; Jiang, L.; Liu, K.; Lü, P.; Gao, J.; Zhang, C. The RhHB1/RhLOX4 module affects the dehydration tolerance of rose flowers (Rosa hybrida) by fine-tuning jasmonic acid levels. Hortic. Res. 2020, 7, 74. [Google Scholar] [CrossRef]
- Allen, B.L.; Taatjes, D.J. The Mediator complex: A central integrator of transcription. Nat. Rev. Mol. Cell Biol. 2015, 16, 155–166. [Google Scholar] [CrossRef]
- Buendía-Monreal, M.; Gillmor, C.S. Mediator: A key regulator of plant development. Dev. Biol. 2016, 419, 7–18. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Qu, L.J. Plant Mediator complex and its critical functions in transcription regulation. J. Integr. Plant Biol. 2016, 58, 106–118. [Google Scholar] [CrossRef]
- Chen, J.; Yang, S.; Fan, B.; Zhu, C.; Chen, Z. The mediator complex: A central coordinator of plant adaptive responses to environmental stresses. Int. J. Mol. Sci. 2022, 23, 6170. [Google Scholar] [CrossRef]
- Li, S.; Xu, J.; Cao, Y.; Wu, J.; Liu, Q.; Zhang, D. Genome-Wide Analyses of CCHC Family Genes and Their Expression Profiles under Drought Stress in Rose (Rosa chinensis). Int. J. Mol. Sci. 2024, 25, 8983. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, S.; Ren, Y.; You, Y.; Wang, Z.; Zhang, Y.; Zhu, X.; Hu, P. Genome-wide identification of HSP90 gene family in Rosa chinensis and its response to salt and drought stresses. 3 Biotech 2024, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhao, J.; Huang, W.; Liu, C.; Hao, X.; Gao, C.; Deng, M.; Wen, J. Genome-Wide Identification of WRKY Transcription Factor Family in Chinese Rose and Response to Drought, Heat, and Salt Stress. Genes 2024, 15, 800. [Google Scholar] [CrossRef] [PubMed]
- Dar, R.A.; Nisar, S.; Tahir, I. Ethylene: A key player in ethylene sensitive flower senescence: A review. Sci. Hortic. 2021, 290, 110491. [Google Scholar] [CrossRef]
- Ichimura, K.; Mizoguchi, T.; Yoshida, R.; Yuasa, T.; Shinozaki, K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000, 24, 655–665. [Google Scholar] [CrossRef]
- Tsugama, D.; Liu, S.; Takano, T. Drought-induced activation and rehydration-induced inactivation of MPK6 in Arabidopsis. Biochem. Biophys. Res. Commun. 2012, 426, 626–629. [Google Scholar] [CrossRef]
- Xu, J.; Chua, N.H. Dehydration stress activates Arabidopsis MPK6 to signal DCP1 phosphorylation. EMBO J. 2012, 31, 1975–1984. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Wang, Q.; Feng, M.; Li, Y.; Meng, Y.; Zhang, Y.; Liu, G.; Ma, Z.; Wu, H. RhMKK9, a rose MAP KINASE KINASE gene, is involved in rehydration-triggered ethylene production in rose gynoecia. BMC Plant Biol. 2017, 17, 51. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Xu, H.W. Arbuscular mycorrhizae improves low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil 2010, 331, 129–137. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought tolerance: Role of organic osmolytes, growth regulators, and mineral nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment, 1st ed.; Parvaiz Ahmad, M.R.W., Ed.; Springer Science+Business Media: New York, NY, USA, 2014; Volume 1, pp. 25–55. [Google Scholar]
- Behrooz, A.; Vahdati, K.; Rejali, F.; Lotfi, M.; Sarikhani, S.; Leslie, C. Arbuscular mycorrhiza and plant growth-promoting bacteria alleviate drought stress in walnut. HortScience 2019, 54, 1087–1092. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Ben-Laouane, R.; Douira, A.; El Modafar, C.; Mitsui, T.; Wahbi, S.; Meddich, A. Arbuscular mycorrhizal fungi mediate drought tolerance and recovery in two contrasting carob (Ceratonia siliqua L.) ecotypes by regulating stomatal, water relations, and (in) organic adjustments. Plants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Zobel, M.; Öpik, M. Plant and arbuscular mycorrhizal fungal (AMF) communities–which drives which? J. Veg. Sci. 2014, 25, 1133–1140. [Google Scholar] [CrossRef]
- Reynolds, W.; Drury, C.; Yang, X.; Tan, C.; Yang, J. Impacts of 48 years of consistent cropping, fertilization and land management on the physical quality of a clay loam soil. Can. J. Soil Sci. 2014, 94, 403–419. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Zou, Y.-N. Arbuscular mycorrhizal fungi and tolerance of drought stress in plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Singapore, 2017; pp. 25–41. [Google Scholar]
- Ren, A.-T.; Zhu, Y.; Chen, Y.-L.; Ren, H.-X.; Li, J.-Y.; Abbott, L.K.; Xiong, Y.-C. Arbuscular mycorrhizal fungus alters root-sourced signal (abscisic acid) for better drought acclimation in Zea mays L. seedlings. Environ. Exp. Bot. 2019, 167, 103824. [Google Scholar] [CrossRef]
- Zhang, M.C.; Duan, L.S.; Zhai, L.X.; Li, J.M.; Tian, X.L.; Wang, B.M.; He, Z.P.; Li, Z.H. Effect of plant growth regulators on water deficit induced yield loss in soybean. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Augé, R.M.; Foster, J.G.; Loescher, W.H.; Stodola, A.J. Symplastic molality of free amino acids and sugars in Rosa roots with regard to VA mycorrhizae and drought. Symbiosis 1992, 12, 1–17. [Google Scholar]
- Pinior, A.; Grunewaldt-Stöcker, G.; von Alten, H.; Strasser, R.J. Mycorrhizal impact on drought stress tolerance of rose plants probed by chlorophyll a fluorescence, proline content and visual scoring. Mycorrhiza 2005, 15, 596–605. [Google Scholar] [CrossRef]
- Augé, R.M.; Schekel, K.A.; Wample, R.L. Osmotic adjustment in leaves of VA mycorrhizal and nonmycorrhizal rose plants in response to drought stress. Plant Physiol. 1986, 82, 765–770. [Google Scholar] [CrossRef]
- Augé, R.M.; Schekel, K.A.; Wample, R.L. Greater leaf conductance of well-watered VA mycorrhizal rose plants is not related to phosphorus nutrition. New Phytol. 1986, 103, 107–116. [Google Scholar] [CrossRef]
- Green, C.D.; Stodola, A.; Augé, R.M. Transpiration of detached leaves from mycorrhizal and nonmycorrhizal cowpea and rose plants given varying abscisic acid, pH, calcium, and phosphorus. Mycorrhiza 1998, 8, 93–99. [Google Scholar] [CrossRef]
- Tripathy, L.; Dash, S.; Giri, T. Impact of different mulch materials on growth and flowering of rose cv. Mainu parle. J. Crop Weed 2019, 15, 28–31. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Poree, F.; Schaeufele, R.; Helmke, H.; Frackenpohl, J.; Lehr, S.; von Koskull-Döring, P.; Christmann, A.; Schnyder, H.; et al. Abscisic acid receptors and coreceptors modulate plant water use efficiency and water productivity. Plant Physiol. 2019, 180, 1066–1080. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Nadira, U.A.; Bibi, N.; Zhang, G.; Wu, F. Tolerance to Combined Stress of Drought and Salinity in Barley. In Combined Stresses in Plants: Physiological, Molecular, and Biochemical Aspects; Mahalingam, R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 93–121. [Google Scholar]
- Monteiro, J.; Nell, T.A.; Barrett, J. Postproduction of potted miniature rose: Flower respiration and single flower longevity. J. Am. Soc. Hortic. Sci. 2001, 126, 134–139. [Google Scholar] [CrossRef]
- Ashkavand, P.; Zarafshar, M.; Tabari, M.; Mirzaie, J.; Nikpour, A.; Bordbar, S.K.; Struve, D.; Striker, G.G. Application of SiO2 nanoparticles as pretreatment alleviates the impact of drought on the physiological performance of Prunus mahaleb (Rosaceae). Boletín Soc. Argent. Botánica 2018, 53, 1–10. [Google Scholar] [CrossRef]
- Kandhol, N.; Jain, M.; Tripathi, D.K. Nanoparticles as potential hallmarks of drought stress tolerance in plants. Physiol. Plant 2022, 174, e13665. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Hasan, M.M.; Hammami, I.; Alghamdi, A.I.; Alshehri, D.; Alatawi, H.A. Green synthesized metal oxide nanoparticles mediate growth regulation and physiology of crop plants under drought stress. Plants 2021, 10, 1730. [Google Scholar] [CrossRef]
- Siddiqui, H.; Ahmed, K.B.M.; Sami, F.; Hayat, S. Silicon nanoparticles and plants: Current knowledge and future perspectives. In Sustainable Agriculture Reviews 41: Nanotechnology for Plant Growth and Development; Springer: Cham, Switzerland, 2020; pp. 129–142. [Google Scholar]
- Wang, H.-Z.; Zhang, L.-H.; Ma, J.; Li, X.-Y.; Li, Y.; Zhang, R.-P.; Wang, R.-Q. Effects of water stress on reactive oxygen species generation and protection system in rice during grain-filling stage. Agric. Sci. China 2010, 9, 633–641. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Kouna, T.; Antonopoulou, C.-I.; Therios, I. Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul. 2011, 65, 315–325. [Google Scholar] [CrossRef]
- Ahmed, M.; Asif, M.; Hassan, F.-u. Augmenting drought tolerance in sorghum by silicon nutrition. Acta Physiol. Plant. 2014, 36, 473–483. [Google Scholar] [CrossRef]
- Keller, C.; Rizwan, M.; Davidian, J.-C.; Pokrovsky, O.; Bovet, N.; Chaurand, P.; Meunier, J.-D. Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta 2015, 241, 847–860. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Qin, H.; Ding, H.; Li, Y.; Guo, T. Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. J. Plant Growth Regul. 2016, 35, 1–10. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Pessarakli, M. Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J. Plant Nutr. 2016, 39, 1001–1015. [Google Scholar] [CrossRef]
- Hassan, F.; Ali, E.; Alamer, K. Exogenous application of polyamines alleviates water stress-induced oxidative stress of Rosa damascena Miller var. trigintipetala Dieck. South Afr. J. Bot. 2018, 116, 96–102. [Google Scholar] [CrossRef]
- Moftah, A.E.; Al-Humaid, A.I. Effects of Kaolin and Pinolene film-forming polymers on water relations and photosynthetic rate of tuberose (Polianthes tuberosa L.) plants under water deficit conditions. J. Appl. Hortic. 2004, 6, 16–22. [Google Scholar] [CrossRef]
- Giday, H.; Fanourakis, D.; Kjaer, K.H.; Fomsgaard, I.S.; Ottosen, C.-O. Foliar abscisic acid content underlies genotypic variation in stomatal responsiveness after growth at high relative air humidity. Ann. Bot. 2013, 112, 1857–1867. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef]
- Kettlewell, P.S.; Heath, W.L.; Haigh, I.M. Yield enhancement of droughted wheat by film antitranspirant application: Rationale and evidence. Agric. Sci. 2010, 1, 143. [Google Scholar] [CrossRef][Green Version]
- Fanourakis, D.; Giday, H.; Li, T.; Kambourakis, E.; Ligoxigakis, E.K.; Papadimitriou, M.; Strataridaki, A.; Bouranis, D.; Fiorani, F.; Heuvelink, E.; et al. Antitranspirant compounds alleviate the mild-desiccation-induced reduction of vase life in cut roses. Postharvest Biol. Technol. 2016, 117, 110–117. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Perik, R.J.; Belde, P.J.M. Effects of surfactants on the longevity of dry-stored cut flowering stems of rose, Bouvardia, and Astilbe. Postharvest Biol. Technol. 1993, 3, 69–76. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Abadie, P.; Belde, P.J.M. Alkylethoxylate surfactants for rehydration of roses and Bouvardia flowers. Postharvest Biol. Technol. 2002, 24, 327–333. [Google Scholar] [CrossRef]
- Younis, A.; Bhatti, M.Z.M.; Riaz, A.; Tariq, U.; Arfan, M.; Nadeem, M.; Ahsan, M. Effect of different types of mulching on growth and flowering of Freesia alba cv. Aurora. Pak. J. Agric. Sci. 2012, 49, 429–433. [Google Scholar]
- Horo, P.; Kullu, N.; Hembrom, P.; Minz, A. Effect of mulching in rose (Rosa hybrida) cv. Maine parle. J. Pharmacogn. Phytochem. 2018, 7, 2415–2417. [Google Scholar]
- Sardar, H.; Akhtar, G.; Akram, A.; Naseem, K. Influence of mulching materials on soil conditions, weeds, plant growth and flower yield of Rosa centifolia. Int. J. Agric. Appl. Sci 2016, 8, 64–71. [Google Scholar]


| Cultivar | Hormonal Changes | Key Findings | References |
|---|---|---|---|
| R. rubiginosa (Sweet briar) | ABA ↑ (3-fold increase). | Prolonged DS contributes to the accumulation of ABA, which is essential in the stress response. | [106] |
| GA3, GA4, GA5, GA6 ↑ (329.8% increase). GA7, GA8, GA9 ↓ (65.5% decrease). Kinetin riboside ↑ (136.2% increase). | High concentrations of kinetin riboside may be associated with reduced growth and increased tolerance. Decreased levels of some gibberellins may enhance the ability of rose plants to withstand DS by inhibiting growth and reallocating energy toward defense mechanisms. | [107] | |
| R. chinensis Jacq. var. minima Rehd. | ↓ IAA, ↓ Zeatin, ↓ GA (with six-day irrigation intervals and a salinity of 4 dS/m). ↑ ABA (With 4 dS/m salinity). | Salinity and extreme WS reduce IAA and zeatin content. Severe water deficits and salinity stress also reduce GA in combination with longer irrigation intervals. ABA levels increase with higher salinity levels. | [107] |
| R. chinensis Jacq. | ↑ ABA (leaves and roots), ↓ ICA (leaves), ↑ ICA (roots), ↓ IAA (leaves), ↑ IAA (roots, MD), ↓ IAA (roots, SD), ↑ ME-IAA (leaves and roots), ↓ IP (leaves), ↑ IP (roots). | DS induces complex hormonal changes in leaves and roots, with ABA playing a greater role in dehydration responses, particularly in leaves. | [45] |
| R. hybrida cv. Samantha | ↑ JA-Ile (petals). | Increased JA-Ile in petals impairs osmotic adjustment, reducing resistance to water stress. | [108] |
| Genotype | DS Conditions | Enzymes | Activity | References |
|---|---|---|---|---|
| R. hybrida cv. Samantha. | Rose flowers dehydration | SOD and APX | Elevated by WS | [124] |
| R. laevigata | Light and DS | SOD and POD | (+) of both enzymes under light DS. (−) under severe DS. | [134] |
| R. hybrida cv. Samantha. | Rose flower dehydration | SOD and Cu/ZnSOD | (+) SOD and Cu/ZnSOD in dehydration. (−) SOD and Cu/ZnSOD after rehydration. | [125] |
| R. chinensis cv. Old Blush | Gradual drought over (30, 60, 90) days | SOD | (+) SOD activity with increasing DS duration. Maximum SOD activity reached after 90 days of DS treatment. (−) SOD content after re-watering. | [65] |
| R. damascena | 100% FC (control), 50% FC (moderate stress), 25% FC (severe stress) | AChE and LOX. inhibitory enzyme activities | Increased antioxidant and inhibitory enzyme activities in both mild and extreme water shortage conditions. | [37] |
| R. damascene; R. canina | (25%, 50%, 100% FC) | CAT POD SOD | (+) of all three enzymes R. damascena showed higher SOD and CAT activity compared to R. canina. No significant difference in POD activity between the two species. | [75] |
| R. bracteata., R. chinensis., R. rouletii, R. foetida; R. indica L., R. gallica., R. hugonis and cv. Borisphen | Controlled leaf wilting under varying temperatures and humidity. | CAT, POD, PPO | (+) of all three enzymes under wilting conditions. R. rouletii, R. foetida, and R. gallica showed sustained enzyme activity even after recovery, suggesting more severe metabolic disruption. | [135] |
| R. damascena | Different PEG concentrations | TAA | (+) (TAA) | [39] |
| R. hybrida | FI (full irrigation), SDI (moderate DS), PRD1 (severe DS), PRD2 (partial root DS) | CAT and APX | Less activity of APX and CAT in control conditions (FI). Upregulation of these enzymes in response to WS. | [81] |
| R. damascena cv. Kashan 93 | Three distinct WD levels (70, 40, and 10% of available water content). | CAT, POD and APX | The three antioxidant enzymes were activated by a lack of water. | [41] |
| Rose Variety | Severity of DS | DS Resistance | Key Findings | Reference |
|---|---|---|---|---|
| R. damascena Mill. | Mild (50% FC), Severe (25% FC) | Medium | Growth reduced (fresh/dry weight down); Pn and Gs increased 31% and 19% (mild), decreased 55% and 36% (severe); RWC and LWP decreased (72% in severe); chlorophyll b decreased 54% (severe); proline increased 34.6% (severe); soluble sugars up 33.6%. | [40] |
| R. damascena cv. Maragheh | Severe (PEG-induced) | Low | Chlorophyll decreased 30%; K+ and P decreased 56% and 52%; leaf number and area reduced 40%; TAA increased. | [39] |
| R. damascena cv. Kashan | Severe (PEG-induced) | Low | Chlorophyll decreased 41%; K+ and P decreased 47% and 52%; TAA increased. | [39] |
| R. damascena var. trigintipetala | Mild (50% FC), Severe (25% FC) | Medium | Growth reduced; proline increased 34.6% (severe); soluble sugars up 33.6%; SOD and CAT increased; K+ increased; other nutrients decreased. | [37] |
| R. hybrida cv. Charming Black | Severe DS | Low | Shoot length, weight, and leaf area reduced; Pn decreased; fresh weight lower than control; photosynthetic pigments decreased. | [42] |
| R. hybrida cv. Club Nika | Mild (75% irrigation), Moderate (50% irrigation). | Medium | Fresh/dry stem weight decreased 19% (mild) and 36% (moderate); bud sprouting was delayed; no change in photo-synthetic pigments. | [44] |
| R. chinensis cv. Old Blush | Severe (90 days without water) | Medium | Leaves wrinkled after 30 days; severe wilting at 90 days; full recovery after rehydration; SOD peaked at 90 days, decreased after rewatering; MDA peaked at 90 days; lowest LWC and SWC at 90 days. | [65] |
| R. fortuniana | Cyclic DS | High | Superior vegetative growth and leaf area; highest drought tolerance among rootstocks. | [59] |
| R. odorata | Low | Severe reduction in growth; lowest drought tolerance among rootstocks. | ||
| R. multiflora | Medium | Intermediate growth reduction; moderate tolerance. | ||
| R. hybrida cv. Dr. Huey | Medium | Intermediate growth reduction; moderate tolerance. | ||
| R. rugosa | Severe (PEG 5%) | High | Pn and Gs improved; Tr decreased 29.42%; structural adaptations (sunken stomata, low stomatal density); increased MDA and oxygen with DS severity. | [67] |
| R. canina | Mild (50% FC), Severe (25% FC) | Medium | Higher chlorophyll under DS; increased H2O2 and MDA; SOD and CAT increased; proline increased (14.5 mM to 75.5 mM at 25% FC). | [123] |
| R. hybrida cv. Samantha | Severe (dehydration) | Medium | SOD and APX elevated; Cu/ZnSOD increased during dehydration, decreased after rehydration; JA-Ile increased in petals; H2O2 and MDA increased, decreased upon rehydration. | [124,125] |
| R. laevigata | Light (mild), Severe | Medium | SOD and POD increased under mild DS, decreased under severe DS. | [134] |
| R. rubiginosa | (drought in dry sites) | Medium | ABA increased 3-fold; higher soluble carbohydrates in dry sites; slower water loss; decreased photosynthetic activity. | [106] |
| R. chinensis Jacq. var. minima Rehd. | Severe (6-day irrigation intervals + salinity) | Low | IAA, zeatin, and GA decreased; ABA increased with higher salinity and severe growth reduction. | [107] |
| R. roxburghii (Gui 2 and Gui 7) | DS | Medium | Nitrogen and P decreased under DS; no change in K+; proline and soluble sugars increased. | [93] |
| Flowers | Dehydration Treatment | Harvest Stage | Water Relations | Flowers Longevity | References |
|---|---|---|---|---|---|
| R. hybrida cv. Wild look | Exposure to air for 3 h | Commercial stage | Enhanced transpiration in dark conditions reduced water uptake. | Accelerated bent neck and stimulated petal abscission. Decreased flower quality. | [163] |
| R. hybrida cv. Samantha | Dehydration for 60 h | Completely opened bud | Increased fresh weight loss | 29% shorter vase life. Irregular flower opening and loss of market quality. | [125] |
| Air-drying for 24 h | Completely opened bud | The water potential declined from −0.5 MPa to −3.4 MPa. The flowers experienced a significant reduction in weight, losing 25.3% of their freshness within 24 h. | Flowers wilting and neck bending. The flowers are vertically compressed and have a low flower height-to-diameter ratio. | [159] | |
| Dehydration for 24 h | Completely opened bud | Flowers’ initial weight was reduced by 22.8%. The water potential dropped from −0.5 MPa to −3.2 MPa. | Petal cell expansion was inhibited. Abnormal flower openings and shapes. | [161] | |
| Cyclic dehydration in the air for 48 h | Completely opened bud | Variations in fresh weight were reflected in variations in water potential. The fresh weight experienced a linear decrease, resulting in a loss of approximately 20%. During the first hour of rehydration, flowers abs-orb water very quickly, returning to their initial fresh weight in about 3 h. | Flower stems are bent, and petals are noticeably withered. Petal expansion inhibition. Abnormal flower opening. Decreased market quality | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarif, H.; Fan, C.; Yuan, G.; Zhou, R.; Chang, Y.; Sun, J.; Lu, J.; Liu, J.; Wang, C. Drought Stress in Roses: A Comprehensive Review of Morphophysiological, Biochemical, and Molecular Responses. Int. J. Mol. Sci. 2025, 26, 4272. https://doi.org/10.3390/ijms26094272
Zarif H, Fan C, Yuan G, Zhou R, Chang Y, Sun J, Lu J, Liu J, Wang C. Drought Stress in Roses: A Comprehensive Review of Morphophysiological, Biochemical, and Molecular Responses. International Journal of Molecular Sciences. 2025; 26(9):4272. https://doi.org/10.3390/ijms26094272
Chicago/Turabian StyleZarif, Hmmam, Chunguo Fan, Guozhen Yuan, Rui Zhou, Yufei Chang, Jingjing Sun, Jun Lu, Jinyi Liu, and Changquan Wang. 2025. "Drought Stress in Roses: A Comprehensive Review of Morphophysiological, Biochemical, and Molecular Responses" International Journal of Molecular Sciences 26, no. 9: 4272. https://doi.org/10.3390/ijms26094272
APA StyleZarif, H., Fan, C., Yuan, G., Zhou, R., Chang, Y., Sun, J., Lu, J., Liu, J., & Wang, C. (2025). Drought Stress in Roses: A Comprehensive Review of Morphophysiological, Biochemical, and Molecular Responses. International Journal of Molecular Sciences, 26(9), 4272. https://doi.org/10.3390/ijms26094272






